Titanium ethoxide
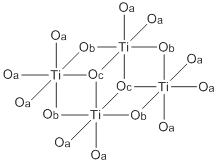 Abbreviated structure of titanium(IV) ethoxide. The ethoxide ligands are represented by O's. The terminal ethoxide ligands are designated by Oa, the doubly bridging ligands by Ob, and the triply bridging ligands by Oc.
| |
| Names | |
|---|---|
| IUPAC name
ethanolate; titanium(4+)
| |
| Systematic IUPAC name
titanium(4+) tetraethanolate | |
| Other names
Ethyl titanate, tetraethyl titanate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.019.464 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C32H80O16Ti4 | |
| Molar mass | 228.109 g/mol |
| Appearance | colorless liquid |
| Density | 1.088 |
| Boiling point | 150–152 (@10 mmHg) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a colorless liquid that is soluble in organic solvents but hydrolyzes readily. It is sold commercially as a colorless solution. Alkoxides of titanium(IV) and zirconium(IV) are used in organic synthesis and materials science. They adopt more complex structures than suggested by their empirical formulas.[1]
Syntheses
Titanium ethoxide is prepared by treating titanium tetrachloride with ethanol in the presence of an amine:[2]
- TiCl4 + 4 EtOH + 4 Et3N → Ti(OEt)4 + 4 Et3NHCl
The purity of titanium ethoxide is commonly assayed by proton NMR spectroscopy. Zr(OEt)4 1H NMR (60 MHz, benzene-d6, ppm): 8.64 (triplet, 12H, CH3), 5.73 (quartet, 8H, CH2).[3] Ti(OEt)4 1H NMR (90 MHz, chloroform-d, ppm): 4.36 (quartet, 8H, CH2), 1.27 (triplet, 12H, CH3).[4]
Structure
Both Ti(OEt)4 exist mainly as tetramers with an octahedral coordination environment around the metal centers There are two types of titanium centers, depending on the number of terminal vs bridging alkoxide ligands. Zr(OEt)4 is structurally similar.[2][5] Zirconium ethoxide is sold commercially as a mixture of the trimer and the tetramer.[6] The virtual symmetry of the M4O16 core structure for the tetramer structures of these compounds is C2h.[7]
Related compounds
Titanium methoxide
Like the ethoxide, titanium methoxide Ti(OMe)4 exists as a tetramer with each of the TiIV metal centers having an octahedral coordination environment.[8]
Titanium isopropoxide
With bulky alky groups, Ti(OiPr)4 in contrast exist as a monomer with a tetrahedral environment around the Ti center. This lower degree of coordination to the metal center is attributed to the steric bulk of the iPr groups versus the n-alkyl groups, this serves to prevent bridging interactions between the metal centers.[9]
Zirconium ethoxide
Zirconium ethoxide can be prepared in a manner similar but not identical to the titanium compound:[10]
- ZrCl4 + 5 NaOEt + EtOH → NaH[Zr(OEt)6] + 4 NaCl
- NaH[Zr(OEt)6] + HCl → Zr(OEt)4 + NaCl + 2 EtOH
A more common synthesis for zirconium ethoxide is to treat zirconium tetrachloride with the desired alcohol and ammonia:[10]
- ZrCl4 + 4 ROH + 4 NH3 → Zr(OR)4 + 4 NH4Cl
Zirconium ethoxide can also be prepared with zirconocene dichloride:[3]
- Cp2ZrCl2 + 4 EtOH + 2 Et3N → 2 CpH + 2 Et3NHCl + Zr(OEt)4
Zirconium propoxide
Zr(OnPr)4 also adopts the titanium ethoxide structure.[5][7]
Reactions
Both Ti and Zr alkoxides can be used to deposit microstructured films of TiO2 or ZrO2:
- M(OEt)4 + 2 H2O → MO2 + 4 HOEt
These films form via a hydrolysis of the alkoxide at a surface interface.[11] It is important to note that the TiO2 and ZrO2 formed by these reactions have a polymeric structure which is where their utility as waterproofing, scratch resistant or heat resistant coatings comes from.[11] The structure of the metal oxide films grown in this matter is affected by the presence of base or acid catalysts for the hydrolysis. Generally acid-catalysis yields a sol where the polymer chains are randomly oriented and linear. In the base-mediated case bushy clusters or crosslinked networks are produced, these structures can trap solvent and reaction byproducts and form a gel coating.[12] TiIV and ZrIV alkoxides are also potential starting materials for Ziegler–Natta catalysts used in olefin polymerization.[11]
References
- ^ Mehrotra, Ram C.; Singh, Anirudh (1997). "Recent Trends in Metal Alkoxide Chemistry". In Karlin, Kenneth D. (ed.). Progress in Inorganic Chemistry. Vol. 46. John Wiley & Sons. pp. 239–454. doi:10.1002/9780470166475.ch4. ISBN 9780470167045.
- ^ a b Cotton, F. A.; Wilkinson, G.; Murillo, C.; Bochmann, M. (1999). Advanced Inorganic Chemistry (6th ed.). New York: John Wiley & Sons. ISBN 978-0-471-19957-1.
- ^ a b Gray, Donald R.; Brubaker, Carl H. (1971). "Preparation and characterization of a series of chloroalkoxobis(cyclopentadienyl)zirconium(IV) and dialkoxobis(cyclopentadienyl)zirconium(IV) compounds". Inorg. Chem. 10 (10): 2143–2146. doi:10.1021/ic50104a010.
- ^ Integrated Spectral Database System of Organic Compounds, version 2011. AIST: Japan, 2011 (accessed October 3rd, 2011).
- ^ a b Ibers, James A. (1963). "Crystal and Molecular Structure of Titanium(IV) Ethoxide". Nature. 197 (4868): 686–687. Bibcode:1963Natur.197..686I. doi:10.1038/197686a0.
- ^ Evans, William J.; Ansari, Mohammad A.; Ziller, Joseph W. (1999). "Synthesis of Zirconium Aryloxide Complexes Containing Pendent Vinyl Groups". Inorg. Chem. 38 (6): 1160–1164. doi:10.1021/ic981201v. PMID 11670898.
- ^ a b Day, Victor W.; Klemperer, Walter G.; Pafford, Margaret M. (2001). "Isolation and Structural Characterization of Tetra-n-propyl Zirconate in Hydrocarbon Solution and the Solid State". Inorg. Chem. 40 (23): 5738–5746. doi:10.1021/ic010776g. PMID 11681880.
- ^ Wright, D. A.; Williams, D. A. (1968). "The Crystal and Molecular Structure of Titanium Tetramethoxide". Acta Crystallographica B. 24 (8): 1107–1114. doi:10.1107/S0567740868003766.
- ^ Ghosh, Rajshekhar; Nethaji, Munirathinam; Samuelson, Ashoka G. (2005). "Reversible double insertion of aryl isocyanates into the Ti–O bond of titanium(IV) isopropoxide". J. Organomet. Chem. 690 (5): 1282–1293. doi:10.1016/j.jorganchem.2004.11.038.
- ^ a b Bradley, D. C.; Wardlaw, W. (1951). "Zirconium alkoxides". J. Chem. Soc.: 280–285. doi:10.1039/jr9510000280.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Schubert, U. (2003). "Sol–Gel Processing of Metal Compounds". In McCleverty, J. A.; Meyer, T. J. (eds.). Comprehensive Coordination Chemistry II. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Vol. 7. Pergamon. pp. 629–656. doi:10.1016/B0-08-043748-6/06213-7. ISBN 978-0-12-409547-2.
