User:JasWeimimi78/sandbox
Light-emitting diode therapy (LEDT)[edit]

Light-emitting diode therapy (LEDT) is a clinical approach that applies different wavelengths of light to cure diseases or conditions with skin-safe lights. Following NASA's innovation in the 1990s with Light Emitting Diodes (LEDs) that emit a specific narrow light spectrum, LED Therapy (LEDT) showed significant potential[1]. The high precision of narrow-band LED therapy enabled its first use in clinical practices. The commonly used lights in LEDT are blue, red, green, yellow, and infrared (IR)[1].
LEDT's general mechanism is related to cellular receptor metabolism. Light functions as an external stimulus and influences cellular metabolism by initiating photo-biochemical reactions within cells. Light Emitting Diode Therapy (LEDT) encompasses two primary therapeutic approaches: photodynamic Therapy (PDT) and photobiomodulation Therapy (PBMT). Photodynamic therapy (PDT) utilises light-sensitive compounds combined with LED light to generate reactive oxygen species, which selectively target and destroy abnormal cells. Oncology and certain skin conditions widely use this technique. Whereas photobiomodulation therapy (PBMT) utilizes low-level LED light to stimulate cellular repair, stimulate wound healing, and reduce inflammation, without the use of photosensitizing agents.
Different wavelengths and mechanisms are utilized for different therapeutic effects. The therapeutic advantages of LED therapy stem from its effectiveness in various treatments, including wound healing, acne treatment, sunburn protection, and the use of phototherapy for facial wrinkles and skin revitalization[2].
Compared to laser phototherapy, Light Emitting Diode Therapy (LEDT) is recognized for its enhanced safety profile, exhibiting fewer short-term and long-term side effects. This distinction stems from LEDT's use of non-coherent light at lower intensities, which minimizes the risks of tissue damage and discomfort often associated with the high-intensity, coherent light of lasers. Still, there are some side effects that can be commonly seen after exposure to light, that vary on the therapy patients take, PBMT or PDT.
History[edit]
The history of light therapy can be traced back to ancient Egypt and India, where therapy with natural sunlight was first used to treat leucoderma.[3] In the 1850s, Florence Nightingale’s advocacy of exposure to clean air and sunlight for health restoration also contributed to the initial development of light therapy for treatments.[4] Later, Downes and Blunt’s experiment in 1877 suggested sunlight’s effect on fungal growth inhibition, which further evidenced the efficiency of light therapy . [5]
The modern use of light therapy with artificial lights started in the late 19th century. The Danish Nobel Prize winner in medicine and physiology, Niels Finsen, pioneered the use of light as a therapy for skin tuberculosis (lupus vulgaris).[6] His creation of the light device “Finsen lamp” as a lupus vulgaris treatment marked the beginning of modern light therapy. The application of LED lights in cosmetology became more popular in the 1980s, particularly for acne recovery, due to its ability of collagen production. Since the early 2000s, the use of LED light therapy in the medical field has become more versatile, including treatment for skin conditions, chronic diseases, and the realignment of human circadian rhythm. It has now become a commonly used therapy in both the beauty and medical fields.
Mechanisms[edit]
LEDs are the most utilized optical semiconductor devices that transform electrical energy into light energy[7]. LED therapy utilizes light-emitting diodes to deliver treatments based on mechanisms such as photodynamic Therapy (PDT) and photobiomodulation (PBMT). PDT targets and destroys diseased cells, while PBMT stimulates cellular repair and reduces inflammation. The effectiveness of LED therapy varies with the wavelength of light, allowing for diverse applications in healing, dermatology, and cancer treatment.
Photodynamic (PDT) mechanism[edit]
PDT can induce many cellular pathways, and its main purpose is to induce cell death, either by apoptosis or necrosis[8]. The fundamental process of photodynamic reactions involves three elements: photosensitizers (PS), light of a specific wavelength, and oxygen present in the cell.[9]The interaction of these three components produced a desirable effect inside targeted tissues. Apart from light and oxygen, photosensitizers are compounds designed to absorb light at particular wavelengths during therapy, which enables them to initiate therapeutic processes.[8]
Two pathway branches interact and contribute to different ratios during PDT therapy, affecting its efficiency. Both mechanisms have the same first stage. When cells absorb photosensitizers and are exposed to light that aligns with their absorption spectrum, these substances photo-excite from their stable ground state (S°) to an energised excited singlet state (S1).[8] Some energy is emitted as fluorescence, remaining energy directs the photosensitizer molecule into triplet state T1.[8]
Type I pathway of photodynamic reaction: T1 state PS can then interact with nearby molecules.[8] Energy transfers between the photosensitizer and other molecules in the form of hydrogen and electrons, resulting in the creation of free radicals and anion radicals. These radicals remain in ground state and react with oxygen, leading to the formation of reactive oxygen species (ROS) and superoxide anion radicals (O2•−), which further react with oxygen to generate ROS.[8]The chain of reactions that generate reactive oxygen species (ROS) results in oxidative stress, which ultimately causes cell damage.[7][8]
Type II pathway of photodynamic reaction: After PS is excited into T1 state, the energy is transferred directly between PS and O2. This interaction transforms the oxygen molecules into a highly reactive form known as singlet oxygen. Singlet oxygen possesses potent oxidizing properties, making it extremely effective in reacting with and damaging cellular components[7]. However, this reaction is selective; while most cellular components exist in a less reactive singlet state and remain unaffected, the singlet oxygen specifically targets and reacts with oxygen molecules in the cell cytoplasm.
PDT is used to treat cancer across diverse types and sites. A unique feature of PDT is that photosensitizers tend to accumulate more selectively in cancer cells than in normal cells.[9] [10]The high affinity of photosensitizers to low-density lipoprotein (LDL) allows for selectivity.[7][8] LDL acts as a carrier, allowing photosensitizers to travel into cancerous tissues. The interaction between photosensitizers and LDL makes it easier for therapeutic agents to reach the right places, which makes PDT more effective against cancer.
Photo-biomodulation therapy (PBMT) mechanism[edit]
Photobiomodulation therapy (PBMT) uses low-power densities and is characterized by its non-heat producing effects, a feature previously associated only with laser light.[11] Nowadays, low-level LED lights offer a cost-effective alternative, expanding the accessibility and application of this therapeutic approach.
PBMT (low-level light) targets mitochondria and has impacts on, 1) raising ROS levels, 2) creating adenosine triphosphate (ATP); and 3) helping to turn on transcription factor.[12] That can trigger biochemical change within the cells, involve photon emitting light absorbed by the photoreceptor and cascade reaction. When exposed to LED light, the cytochrome c oxidase (CCO) inside the electron transport chain (ETC) of mitochondria is targeted.[12] Its two heme and two copper subunits are oxidized or reduced, enabling it to absorb light at various wavelengths. CCO is the main target of near-infrared and red(650-1000nm) wavelengths.
Cytochrome c oxidase (CCO) is a key protein in the Electron Transport Chain, responsible for transferring electrons to the final oxygen acceptor. This action helps build a substantial proton gradient across the inter-membrane space of mitochondria; a process critical for the synthesis of ATP (Adenosine Triphosphate). The increased production of ATP because of this activity. CCO is also a photoreceptor, the photon absorption of CCO can lead to enhanced enzyme activity, increased oxygen consumption and usage of ATP production and the release of NO (nitric oxide).[12]
The NO can then contribute to two suggested pathways. Under typical conditions, nitric oxide (NO) interacts non-covalently with the heme and copper (Cu) subunits of CCO, competing with oxygen molecules and inhibiting cellular respiration, leading to reduced production of adenosine triphosphate (ATP).[12] Low energy light can revise the mitochondrial inhibition of cellular respiration by photodissociating of NO from CCO, and thereby increasing ATP synthesis.[13]
The second pathway proposes that released nitric oxide (NO) will boost Cytochrome c Oxidase (CCO) activity, as an enzyme nitrite reductase.[12][13] This mechanism includes the transfer of electrons to oxygen molecules, resulting in the production of water and reactive oxygen species (ROS).[12] The ROS then activates enzymes necessary for producing vital cellular components like nucleic acids and proteins.[12] LED therapy may also increase ROS, which may activate transcription factors that manage genes important for cell growth, cytokine production, and making growth factors for cell repair and proliferation.[13]
Wavelength[edit]

LEDs produce wavelengths that span from UV-A (350 nm) to near-infrared (NIR) (1100 nm)[14]. The wavelength of the LED light can target different tissues. Long wavelength lights such as NIR/dark red(600-1000nm) can have better tissue penetration and can easily absorb cytochrome c oxidase (CCO) targets by PBMT. Therefore, the long wavelength light is used for dermatology and cosmetics applications. While short wavelength light, green or blue light can be absorbed and target hemoglobin in the blood.[14]
Current application of LED light therapy[edit]
Red light therapy, utilising red LED lights, originated from techniques intended to enhance plant growth in space and aid in astronauts' wound healing.[15] Primarily used in dermatology, it enhances skin conditions by stimulating mitochondria, thereby increasing collagen production and blood circulation while reducing inflammation.[15] Additionally, it plays a crucial role in photodynamic therapy, where it combines with photosensitive drugs to target and destroy cancerous cells through a light-induced chemical reaction.
Blue light therapy is also a common LED light therapy to treat acne, skin cancer, and depression.[16] While blue light therapy has similar mechanisms for skin enhancement as red light therapy, its usage for photodynamic therapies in treating cancer are slightly different. Blue light therapy stimulates immune system defences, destroys blood vessels that help cancer cells grow, and causes cell death by reacting with oxygen.[16] It also prevents skin cancer and metastasis by removing precancerous and cancerous skin lesions.[16]
Application of red and blue LED light for photodynamic treatments are generally safe and can be used to treat different cancers. However, they may differ in the efficiency and response.[17] For instance, In the treatment of Gorlin syndrome, a genetic disorder predisposed to cancer, studies have shown that blue light therapy achieves a higher tumor clearance rate and induces less pain than red light therapy.[17][18]
As a result of the increasing popularity of LED light therapies for skin improvements, a wide range of skincare devices utilizing this technology are readily available on the market. LED red light products are most common for domestic LED light therapy, including LED light masks, panels, handheld devices, and belts. Typically, individuals buy these items to address issues like wrinkles and acne, reduce puffiness, and promote hair growth.
Therapeutic Effects[edit]
The ameliorated LEDT represents the emerging and safest therapeutic approach for various dermatological conditions, such as skin inflammatory conditions, aging, and hair growth disorders.
1. Skin inflammatory condition[edit]
Acne vulgaris[edit]

Acne vulgaris is a common chronic inflammatory pilosebaceous skin disorder associated with papules, pustules, or nodules primarily on the face.[20] The separate and combined therapeutic effects of red and blue lights LEDT proved to be effective for acne therapy.
Skin sebum plugs is the most typical cause of Acne. The red-light LEDT is proven to have a significant inhibitory effect on cutaneous fat secretion.[21]
Blue light (at a wavelength of 415 nm) reduces sebum secretion through the inhibiting effect on sebocyte proliferation. In the acne treatment case, blue-light LEDT is conducted in a dose-dependent approach, suggesting that therapeutic progress relies on the dose increments.[19] Besides the direct influence on microorganisms, LEDT can also lead to indirect effects on a microorganism’s density by modulating the immune response.[22]
The combination effects of red and blue lights were also proven to perform inhibitory effects in the therapy of mild-to-moderate inflammatory acne lesions in in-vivo clinical trials.[23]
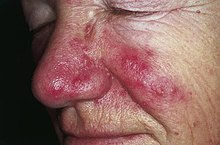
Rosacea[edit]
Rosacea is a chronic skin disorder that presents with recurrent flushing, erythema, papules, and telangiectasia.[24] Methyl aminolevulinate (MAL) is a drug used as a photosensitizer in photodynamic therapy like LEDT. The red-light MAL-LED therapy performed significant therapeutic effects on the recovery of papulopustular lesions in an in-vivo clinical trial conducted with rosacea patients. A more recent study proves the in-vitro effect of LEDT at wavelengths of 630 nm and 940 nm in rosacea-like mouse skin.[25]

2. Anti-aging and rejuvenation treatment[edit]
Solar UV radiation accelerates the skin ageing rate and contributes to abnormal cutaneous status by increasing matrix-metalloproteinase (MMP) expression in photo-damaged skin. MMPs consist of various proteinases that degrade collagen fibrils and other components of the cutaneous extracellular matrix.[26]
The commonly used therapies for skin ageing involve the use of retinoic acid, laser resurfacing, peels (trichloroacetic acid and CO2), injectable skin rejuvenation, and dermal fillers.[27]In coemparison, LEDT is a more non-ablative cutaneous rejuvenation approach with less safety concern and higher effectiveness.
LEDT can reduce MMP expression through the induction of skin collagen synthesis.[28]LECT with yellow LED (at a wavelength of 590 nm) contributes to the skin rejuvenation effects on nearly a thousand clinical samples.[29]
Similarly, red-light (660 nm) LEDT shows curing effects on individuals with photodamaged skin via the reverse of the collagen down-regulation mechanism and MMP expression up-regulation.[30] Researchers conclude that the use of red-light LEDT is an efficient collagen enhancement strategy. LEDT's combination therapy of different light wavelengths also shows greater efficacy. For instance, blue-light LEDT coupled with photosensitizers reveals better elasticity, less pigmentation, and more complexion of the skin.[18]
3. Cancerous skin lesions[edit]

The cancerous skin lesions refer to damaged cutaneous tissues with risks of further developing into skin cell carcinoma.[31]Skin carcinoma, or skin cancer, is very common in sun-radiation-abundant areas. The over-exposure to sunlight is the most prevalent cause that leads to Actinic keratosis (AK), a common cancerous cutaneous lesion.
Photodynamic therapy (PDT) has proven to be an effective approach for AK at sites of poor healing with few responses to other therapies.[32] In the comparison study between MAL-LEDT and typical cryotherapy, blue-light LEDT with Aminolevulinic acid (ALA) as a photosensitizer reveals significantly higher healing rates. The other clinical trial illustrates the effect of combined therapy of red-light LEDT and nano-emulsion in treating cancerous skin lesions.[33]
4. Hair loss disorder[edit]
The first LLLT (low-level laser device) device (at a wavelength of 635 nm) for androgenetic alopecia was approved by the FDA two decades ago. The FDA then approved a similar device (at a wavelength of 655 nm) for alopecia two years later, in 2009.
Low-level laser therapy (LLLT) is the traditional FDA-approved therapy for hair loss disorders, while LEDT has become a more advanced treatment due to its safety and efficacy. The commonly used wavelengths targeting hair loss disorder are red and infrared.[34] In-vivo studies show that the red-light LEDT at the wavelength of 655 nm contributes to dramatic improvements in the hair counts of androgenetic alopecia patients.[35] A more recent study indicates a similar therapeutic effect of yellow-light LEDT on androgenetic alopecia and alopecia areata patients.[36]
Side effects[edit]
Side effects of photobiomodulation therapy[edit]
While Photobiomodulation (PBM) therapy is generally considered safe, there are infrequent instances of immediate adverse effects[37]. Common side effects associated with this form of light therapy can include itching, the appearance of red spots, congestion along the deep external auditory canal wall, and mild allergic reactions. These side effects are typically transient and resolve without intervention.[37]
Side effects of photodynamic therapy.[edit]

The side effects of photodynamic therapy can be divided into onset side effects, which occur which early exposure to light[39]. Early onset side effects of photodynamic Therapy (PDT) commonly include pain and Local Skin Reactions (LSRs), such as erythema, Edema, desquamation, and pustulae[39]. These effects are frequently observed during or shortly after exposure to the light source used in PDT and may occur in combination. Less common side effects include urticaria, contact dermatitis, and erosive pustular dermatosis of the scalp (EPDS). Additionally, PDT can have an acute impact on the immune system, which, although immediate in onset, may have long-term implications for treatment-related changes in carcinogenesis.
Late onset side effects, include pigmentary changes and scarring, affecting approximately 0.8% of patients.[39]There is also a risk of developing bullous pemphigoid, and there is potential for PDT to induce or stimulate skin carcinogenesis[39].
LED light therapy v.s laser therapy[edit]
In the field of phototherapy, Low-Level Laser Therapy (LLLT) and LED Therapy (LEDT) are well-known modalities that provide non-invasive treatment options for a variety of medical conditions. Low-Level Laser Therapy (LLLT) employs low-intensity lasers, occasionally supplemented by LED lighting, to address a variety of medical conditions.[40] Similar to LED Therapy (LEDT), LLLT's applications include the treatment of skin issues such as inflammation and pigmentation, tissue damage, and cardiovascular concerns.[40] Although both LEDT and LLLT share therapeutic goals, LEDT is particularly noted for its cost-effectiveness and is designed for broader coverage using expansive LED panels, whereas LLLT utilizes more focused, coherent laser light for targeted areas.[7][40] For this reason, laser therapy is appropriate for treating tissues beneath the hypodermis, LED therapy is more effective in treating cutaneous diseases.[7]
References[edit]
- ^ a b Calderhead, R Glen (2007). "THE PHOTOBIOLOGICAL BASICS BEHIND LIGHT-EMITTING DIODE (LED) PHOTOTHERAPY". LASER THERAPY. 16 (2): 97–108. doi:10.5978/islsm.16.97. ISSN 1884-7269.
- ^ Leal-Junior, Ernesto Cesar Pinto; Vanin, Adriane Aver; Miranda, Eduardo Foschini; de Carvalho, Paulo de Tarso Camillo; Dal Corso, Simone; Bjordal, Jan Magnus (2015-02-01). "Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis". Lasers in Medical Science. 30 (2): 925–939. doi:10.1007/s10103-013-1465-4. ISSN 1435-604X.
- ^ Hönigsmann, Herbert (2012-01-09). "History of phototherapy in dermatology". Photochemical & Photobiological Sciences. 12 (1): 16–21. doi:10.1039/c2pp25120e. ISSN 1474-905X.
- ^ Liebert, Ann; Kiat, Hosen (2021-03-04). "The history of light therapy in hospital physiotherapy and medicine with emphasis on Australia: Evolution into novel areas of practice". Physiotherapy Theory and Practice. 37 (3): 389–400. doi:10.1080/09593985.2021.1887060. ISSN 0959-3985.
- ^ Reed, Nicholas G. (2010). "The History of Ultraviolet Germicidal Irradiation for Air Disinfection". Public Health Reports. 125 (1): 15–27. ISSN 0033-3549. PMC 2789813. PMID 20402193.
- ^ Grzybowski, Andrzej; Pietrzak, Krzysztof (2012-07-01). "From patient to discoverer—Niels Ryberg Finsen (1860–1904)—the founder of phototherapy in dermatology". Clinics in Dermatology. Subcutaneous Mycoses. 30 (4): 451–455. doi:10.1016/j.clindermatol.2011.11.019. ISSN 0738-081X.
- ^ a b c d e f Oh, Phil-Sun; Jeong, Hwan-Jeong (2019-03-01). "Therapeutic application of light emitting diode: Photo-oncomic approach". Journal of Photochemistry and Photobiology B: Biology. 192: 1–7. doi:10.1016/j.jphotobiol.2019.01.003. ISSN 1011-1344.
- ^ a b c d e f g h Robertson, C.A.; Evans, D. Hawkins; Abrahamse, H. (2009-07-01). "Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT". Journal of Photochemistry and Photobiology B: Biology. 96 (1): 1–8. doi:10.1016/j.jphotobiol.2009.04.001.
- ^ a b Kwiatkowski, Stanisław; Knap, Bartosz; Przystupski, Dawid; Saczko, Jolanta; Kędzierska, Ewa; Knap-Czop, Karolina; Kotlińska, Jolanta; Michel, Olga; Kotowski, Krzysztof; Kulbacka, Julita (2018-10-01). "Photodynamic therapy – mechanisms, photosensitizers and combinations". Biomedicine & Pharmacotherapy. 106: 1098–1107. doi:10.1016/j.biopha.2018.07.049. ISSN 0753-3322.
- ^ Chilakamarthi, Ushasri; Giribabu, Lingamallu (2017-08-01). "Photodynamic Therapy: Past, Present and Future". The Chemical Record. 17 (8): 775–802. doi:10.1002/tcr.201600121.
- ^ Sorbellini, Elisabetta; Rucco, Mariangela; Rinaldi, Fabio (2018-09-01). "Photodynamic and photobiological effects of light-emitting diode (LED) therapy in dermatological disease: an update". Lasers in Medical Science. 33 (7): 1431–1439. doi:10.1007/s10103-018-2584-8. ISSN 1435-604X. PMC 6133043. PMID 30006754.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b c d e f g Van Tran, Vinh; Chae, Minhe; Moon, Ju-Young; Lee, Young-Chul (2021-03-01). "Light emitting diodes technology-based photobiomodulation therapy (PBMT) for dermatology and aesthetics: Recent applications, challenges, and perspectives". Optics & Laser Technology. 135: 106698. doi:10.1016/j.optlastec.2020.106698. ISSN 0030-3992.
- ^ a b c de Freitas, Lucas Freitas; Hamblin, Michael R (2016-05-01). "Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy". IEEE Journal of Selected Topics in Quantum Electronics. 22 (3): 348–364. doi:10.1109/JSTQE.2016.2561201. ISSN 1077-260X.
- ^ a b c Lipko, Nancy B. (2022-04-01). "Photobiomodulation: Evolution and Adaptation". Photobiomodulation, Photomedicine, and Laser Surgery. 40 (4): 213–233. doi:10.1089/photob.2021.0145. ISSN 2578-5478.
- ^ a b Ablon, Glynis (2018-02-17). "Phototherapy with Light Emitting Diodes". The Journal of Clinical and Aesthetic Dermatology. 11 (2): 21–27. ISSN 1941-2789. PMC 5843358. PMID 29552272.
- ^ a b c "Photodynamic therapy - Mayo Clinic". www.mayoclinic.org. Retrieved 2024-04-10.
- ^ a b Maytin, Edward V.; Kaw, Urvashi; Ilyas, Muneeb; Mack, Judith A.; Hu, Bo (2018-06-01). "Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: A bilaterally controlled comparison study". Photodiagnosis and Photodynamic Therapy. 22: 7–13. doi:10.1016/j.pdpdt.2018.02.009. ISSN 1572-1000.
- ^ a b Jagdeo, Jared; Austin, Evan; Mamalis, Andrew; Wong, Christopher; Ho, Derek; Siegel, Daniel M. (2018-01-22). "Light‐emitting diodes in dermatology: A systematic review of randomized controlled trials". Lasers in Surgery and Medicine. 50 (6): 613–628. doi:10.1002/lsm.22791. ISSN 0196-8092.
- ^ a b Jung, Yu Ra; Kim, Sue Jeong; Sohn, Kyung Cheol; Lee, Young; Seo, Young Joon; Lee, Young Ho; Whang, Kyu Uang; Kim, Chang Deok; Lee, Jeung Hoon; Im, Myung (2015-02-18). "Regulation of lipid production by light-emitting diodes in human sebocytes". Archives of Dermatological Research. 307 (3): 265–273. doi:10.1007/s00403-015-1547-1. ISSN 0340-3696.
- ^ Yan, Hui‐Min; Zhao, Hui‐Juan; Guo, Du‐Yi; Zhu, Pei‐Qiu; Zhang, Chun‐Lei; Jiang, Wei (2018-08-13). "Gut microbiota alterations in moderate to severe acne vulgaris patients". The Journal of Dermatology. 45 (10): 1166–1171. doi:10.1111/1346-8138.14586. ISSN 0385-2407.
- ^ Smith, K.R.; Thiboutot, D.M. (2008-02-01). "Thematic review series: Skin Lipids. Sebaceous gland lipids: friend or foe?". Journal of Lipid Research. 49 (2): 271–281. doi:10.1194/jlr.r700015-jlr200. ISSN 0022-2275.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Dreno, B.; Gollnick, H.P.M.; Kang, S.; Thiboutot, D.; Bettoli, V.; Torres, V.; Leyden, J. (2015-06-01). "Understanding innate immunity and inflammation in acne: implications for management". Journal of the European Academy of Dermatology and Venereology. 29 (S4): 3–11. doi:10.1111/jdv.13190. ISSN 0926-9959.
- ^ Kwon, H.H.; Lee, J.B.; Yoon, J.Y.; Park, S.Y.; Ryu, H.H.; Park, B.M.; Kim, Y.J.; Suh, D.H. (2013-04-25). "The clinical and histological effect of home-use, combination blue-red LED phototherapy for mild-to-moderate acne vulgaris in Korean patients: a double-blind, randomized controlled trial". British Journal of Dermatology. 168 (5): 1088–1094. doi:10.1111/bjd.12186. ISSN 0007-0963.
- ^ Thiboutot, Diane; Anderson, Rox; Cook-Bolden, Fran; Draelos, Zoe; Gallo, Richard L.; Granstein, Richard D.; Kang, Sewon; Macsai, Marian; Gold, Linda Stein; Tan, Jerry (2020-06-01). "Standard management options for rosacea: The 2019 update by the National Rosacea Society Expert Committee". Journal of the American Academy of Dermatology. 82 (6): 1501–1510. doi:10.1016/j.jaad.2020.01.077. ISSN 0190-9622.
- ^ Lee, Jee‐Bum; Bae, Soo Hyeon; Moon, Ki Rang; Na, Eui Young; Yun, Sook Jung; Lee, Seung‐Chul (2016-10-03). "Light‐emitting diodes downregulate cathelicidin, kallikrein and toll‐like receptor 2 expressions in keratinocytes and rosacea‐like mouse skin". Experimental Dermatology. 25 (12): 956–961. doi:10.1111/exd.13133. ISSN 0906-6705.
- ^ Yaar, M.; Gilchrest, B.A. (2007-11-01). "Photoageing: mechanism, prevention and therapy". British Journal of Dermatology. 157 (5): 874–887. doi:10.1111/j.1365-2133.2007.08108.x. ISSN 0007-0963.
- ^ Ganceviciene, Ruta; Liakou, Aikaterini I.; Theodoridis, Athanasios; Makrantonaki, Evgenia; Zouboulis, Christos C. (2012-07-15). "Skin anti-aging strategies". Dermato-Endocrinology. 4 (3): 308–319. doi:10.4161/derm.22804. ISSN 1938-1980.
- ^ Brondon, Philip; Stadler, Istvan; Lanzafame, Raymond J. (2009-03-15). "Pulsing influences photoradiation outcomes in cell culture". Lasers in Surgery and Medicine. 41 (3): 222–226. doi:10.1002/lsm.20740. ISSN 0196-8092.
- ^ Weiss, Robert A.; McDaniel, David H.; Geronemus, Roy G.; MARGARET, A. WEISS; KAREN, L. BEASLEY; Munavalli, Girish M.; Bellew, Supriya G. (2005-09-27). "Clinical Experience with Light‐Emitting Diode (LED) Photomodulation". Dermatologic Surgery. 31 (s3): 1199–1205. doi:10.1111/j.1524-4725.2005.31926. ISSN 1076-0512.
- ^ Barolet, Daniel; Roberge, Charles J.; Auger, François A.; Boucher, Annie; Germain, Lucie (2009-12-07). "Regulation of Skin Collagen Metabolism In Vitro Using a Pulsed 660nm LED Light Source: Clinical Correlation with a Single-Blinded Study". Journal of Investigative Dermatology. 129 (12): 2751–2759. doi:10.1038/jid.2009.186. ISSN 0022-202X.
- ^ Dodds, Annabel; Chia, Alvin; Shumack, Stephen (2014-03-14). "Actinic Keratosis: Rationale and Management". Dermatology and Therapy. 4 (1): 11–31. doi:10.1007/s13555-014-0049-y. ISSN 2193-8210.
- ^ de Berker, D.; McGregor, J.M.; Hughes, B.R. (2007-02-09). "Guidelines for the management of actinic keratoses". British Journal of Dermatology. 156 (2): 222–230. doi:10.1111/j.1365-2133.2006.07692.x. ISSN 0007-0963.
- ^ Szeimies, R.-M.; Radny, P.; Sebastian, M.; Borrosch, F.; Dirschka, T.; Krähn-Senftleben, G.; Reich, K.; Pabst, G.; Voss, D.; Foguet, M.; Gahlmann, R.; Lübbert, H.; Reinhold, U. (2010-05-28). "Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a prospective, randomized, double-blind, placebo-controlled phase III study". British Journal of Dermatology. 163 (2): 386–394. doi:10.1111/j.1365-2133.2010.09873.x. ISSN 0007-0963.
- ^ Lanzafame, Raymond J.; Blanche, Raymond R.; Chiacchierini, Richard P.; Kazmirek, Eric R.; Sklar, Jeffrey A. (2014-10-07). "The growth of human scalp hair in females using visible red light laser and LED sources". Lasers in Surgery and Medicine. 46 (8): 601–607. doi:10.1002/lsm.22277. ISSN 0196-8092.
- ^ Ganceviciene, Ruta; Liakou, Aikaterini I.; Theodoridis, Athanasios; Makrantonaki, Evgenia; Zouboulis, Christos C. (2012-07-20). "Skin anti-aging strategies". Dermato-Endocrinology. 4 (3): 308–319. doi:10.4161/derm.22804. ISSN 1938-1980.
- ^ Atzmony, Lihi; Reiter, Ofer; Hodak, Emmilia; Gdalevich, Michael; Mimouni, Daniel (2015-10-27). "Treatments for Cutaneous Lichen Planus: A Systematic Review and Meta-Analysis". American Journal of Clinical Dermatology. 17 (1): 11–22. doi:10.1007/s40257-015-0160-6. ISSN 1175-0561.
- ^ a b Nikookam, Yasmin; Zia, Nawal; Lotfallah, Andrew; Muzaffar, Jameel; Davis-Manders, Jennifer; Kullar, Peter; Smith, Matthew E.; Bale, Gemma; Boyle, Patrick; Irving, Richard; Jiang, Dan; Bance, Manohar (2023-11-23). "The effect of photobiomodulation on tinnitus: a systematic review". The Journal of Laryngology & Otology: 1–22. doi:10.1017/S0022215123002165. ISSN 0022-2151.
- ^ Dugan, Elizabeth M.; Huber, Adam M.; Miller, Frederick W.; Rider, Lisa G.; Group, the International Myositis Assessment and Clinical Studies (IMACS) (2009-02-01). "Photoessay of the cutaneous manifestations of the idiopathic inflammatory myopathies". Dermatology Online Journal. 15 (2). doi:10.5070/D31f04d17z.
- ^ a b c d Borgia, Francesco; Giuffrida, Roberta; Caradonna, Emanuela; Vaccaro, Mario; Guarneri, Fabrizio; Cannavò, Serafinella P. (2018-03-10). "Early and Late Onset Side Effects of Photodynamic Therapy". Biomedicines. 6 (1): 12. doi:10.3390/biomedicines6010012. ISSN 2227-9059. PMC 5874669. PMID 29382133.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b c Leal-Junior, Ernesto Cesar Pinto; Vanin, Adriane Aver; Miranda, Eduardo Foschini; de Carvalho, Paulo de Tarso Camillo; Dal Corso, Simone; Bjordal, Jan Magnus (2015-02-01). "Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis". Lasers in Medical Science. 30 (2): 925–939. doi:10.1007/s10103-013-1465-4. ISSN 1435-604X.
