Per- and polyfluoroalkyl substances: Difference between revisions
Added external link |
→Bioaccumulation and biomagnification: updated with 1 item from 2023 in science (freshwater fish), ce |
||
| Line 81: | Line 81: | ||
[[Bioaccumulation]] is the process by which PFASs are transferred into the tissue of any exposed organisms where PFASs accumulate over time since organisms lack natural excretion mechanisms. PFASs can accumulate in marine species by a variety of pathways. They can be absorbed from the environment, such as contaminated sediments or PFASs dissolved in water. PFASs can partition into the organs and tissues of marine organisms from these environmental compartments. They have been shown to bind to blood proteins and accumulate in the livers of marine animals.<ref name="Jones">{{cite journal | vauthors = Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP | title = Binding of perfluorinated fatty acids to serum proteins | journal = Environmental Toxicology and Chemistry | volume = 22 | issue = 11 | pages = 2639–2649 | date = November 2003 | pmid = 14587903 | doi = 10.1897/02-553 | s2cid = 15768654 }}</ref> Another pathway for bioaccumulation is predation. As larger marine animals feed on smaller organisms that have been exposed to PFASs, the larger animals absorb the PFASs contained in their prey. |
[[Bioaccumulation]] is the process by which PFASs are transferred into the tissue of any exposed organisms where PFASs accumulate over time since organisms lack natural excretion mechanisms. PFASs can accumulate in marine species by a variety of pathways. They can be absorbed from the environment, such as contaminated sediments or PFASs dissolved in water. PFASs can partition into the organs and tissues of marine organisms from these environmental compartments. They have been shown to bind to blood proteins and accumulate in the livers of marine animals.<ref name="Jones">{{cite journal | vauthors = Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP | title = Binding of perfluorinated fatty acids to serum proteins | journal = Environmental Toxicology and Chemistry | volume = 22 | issue = 11 | pages = 2639–2649 | date = November 2003 | pmid = 14587903 | doi = 10.1897/02-553 | s2cid = 15768654 }}</ref> Another pathway for bioaccumulation is predation. As larger marine animals feed on smaller organisms that have been exposed to PFASs, the larger animals absorb the PFASs contained in their prey. |
||
[[File:The build up of toxins in a food chain.svg|thumb|'''[[Biomagnification]]''' is the build up of toxins in a food chain. As the trophic level increases in a food chain, the amount of toxic build up increases. The x's represent the amount of toxic build up accumulating as the trophic level increases. Toxins build up in organism's fat and tissue. Predators accumulate higher toxins than prey as a result of bioaccumulation. ]] |
[[File:The build up of toxins in a food chain.svg|thumb|'''[[Biomagnification]]''' is the build up of toxins in a food chain. As the trophic level increases in a food chain, the amount of toxic build up increases. The x's represent the amount of toxic build up accumulating as the trophic level increases. Toxins build up in organism's fat and tissue. Predators accumulate higher toxins than prey as a result of bioaccumulation. ]] |
||
[[Biomagnification]] is the process by which the amount of PFAS contamination increases with increasing [[trophic level]], due to predation by the species higher in the food web. Top predators have higher levels of PFASs than species lower down the food chain. Seabirds that feed on fish have among the highest levels of PFAS contamination.<ref name="Jones" /> [[Perfluorosulfonic acids]], which have a sulfonic acid functional group attached to the fluorinated "tail", have a greater tendency to bioaccumulate than [[Perfluorinated carboxylic acid|perfluorocarboxylic acids]], which contain a carboxylic acid function group. Longer chain PFAS compounds, which have 6, 7, or more fluorinated carbons, bioaccumulate in greater quantities than shorter chain PFAS compounds. |
[[Biomagnification]] is the process by which the amount of PFAS contamination increases with increasing [[trophic level]], due to predation by the species higher in the food web. Top predators have higher levels of PFASs than species lower down the food chain. Seabirds that feed on fish have among the highest levels of PFAS contamination.<ref name="Jones" /> [[Perfluorosulfonic acids]], which have a sulfonic acid functional group attached to the fluorinated "tail", have a greater tendency to bioaccumulate than [[Perfluorinated carboxylic acid|perfluorocarboxylic acids]], which contain a carboxylic acid function group. Longer chain PFAS compounds, which have 6, 7, or more fluorinated carbons, bioaccumulate in greater quantities than shorter chain PFAS compounds. |
||
;In marine species of the food web |
|||
The concentration of PFOS, a long chain sulfonic acid, was found at the highest concentrations relative to other PFASs measured in fish and birds in Northern seas such as the Barents Sea and the Canadian Arctic.<ref>{{cite journal | vauthors = Martin JW, Mabury SA, Solomon KR, Muir DC | title = Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss) | journal = Environmental Toxicology and Chemistry | volume = 22 | issue = 1 | pages = 196–204 | date = January 2003 | pmid = 12503765 | doi = 10.1002/etc.5620220126 | s2cid = 12659454 }}</ref> A study and an interactive map by [[Environmental Working Group|EWG]] using its results showed [[freshwater fish]] in the U.S.{{globalize inline|date=February 2023}} ubiquitously contain high levels of harmful PFAS, with a single serving typically significantly increasing the blood [[Perfluorooctanesulfonic acid|PFOS]] level.<ref>{{cite news |last1=LaMotte |first1=Sandee |title=Locally caught fish are full of dangerous chemicals called PFAS, study finds |url=https://edition.cnn.com/2023/01/17/health/freshwater-fish-pfas-contamination-wellness/index.html |access-date=15 February 2023 |work=CNN |date=17 January 2023 |language=en |archive-date=14 February 2023 |archive-url=https://web.archive.org/web/20230214213701/https://edition.cnn.com/2023/01/17/health/freshwater-fish-pfas-contamination-wellness/index.html |url-status=live }}</ref><ref>{{cite journal |last1=Barbo |first1=Nadia |last2=Stoiber |first2=Tasha |last3=Naidenko |first3=Olga V. |last4=Andrews |first4=David Q. |title=Locally caught freshwater fish across the United States are likely a significant source of exposure to PFOS and other perfluorinated compounds |journal=Environmental Research |date=1 March 2023 |volume=220 |pages=115165 |doi=10.1016/j.envres.2022.115165 |pmid=36584847 |bibcode=2023ER....220k5165B |s2cid=255248441 |language=en |issn=0013-9351}}</ref> |
|||
Bioaccumulation and biomagnification of PFASs in marine species throughout the food web, particularly frequently consumed fish and shellfish, can have important impacts on human populations.<ref>{{cite journal | vauthors = Choi S, Kim JJ, Kim MH, Joo YS, Chung MS, Kho Y, Lee KW | title = Origin and organ-specific bioaccumulation pattern of perfluorinated alkyl substances in crabs | journal = Environmental Pollution | volume = 261 | pages = 114185 | date = June 2020 | pmid = 32114125 | doi = 10.1016/j.envpol.2020.114185 | s2cid = 211727091 }}</ref> PFASs have been frequently documented in both fish and shellfish that are commonly consumed by human populations.<ref name="Perfluoroalkyl substances PFASs i">{{cite journal | vauthors = Fair PA, Wolf B, White ND, Arnott SA, Kannan K, Karthikraj R, Vena JE | title = Perfluoroalkyl substances (PFASs) in edible fish species from Charleston Harbor and tributaries, South Carolina, United States: Exposure and risk assessment | journal = Environmental Research | volume = 171 | pages = 266–277 | date = April 2019 | pmid = 30703622 | pmc = 6943835 | doi = 10.1016/j.envres.2019.01.021 | bibcode = 2019ER....171..266F }}</ref> This poses health risks to humans and studies on the bioaccumulation in certain species are important to determine daily tolerable limits for human consumption, and where those limits may be exceeded causing potential health risks.<ref>{{Cite journal | vauthors = Teunen L, Bervoets L, Belpaire C, De Jonge M, Groffen T |date=2021-03-29 |title=PFAS accumulation in indigenous and translocated aquatic organisms from Belgium, with translation to human and ecological health risk |journal=Environmental Sciences Europe |volume=33 |issue=1 |pages=39 |doi=10.1186/s12302-021-00477-z |s2cid=232414650 |issn=2190-4715}}</ref> This has particular implications for populations that consume larger numbers of wild fish and shellfish species.<ref name="Perfluoroalkyl substances PFASs i"/> In addition to health risks, populations may be impacted by advisories, limits of closures of fishing for certain species that are put in place to help mitigate health risks from potential consumption of species with higher levels of accumulated PFASs, but result in a loss of food sources and important subsistence species depended on by local communities. There is much research being done in this area, including into spatial patterns of PFAS bioaccumulation in fish and crustaceans<ref>{{cite journal | vauthors = Taylor MD, Beyer-Robson J, Johnson DD, Knott NA, Bowles KC | title = Bioaccumulation of perfluoroalkyl substances in exploited fish and crustaceans: Spatial trends across two estuarine systems | journal = Marine Pollution Bulletin | volume = 131 | issue = Pt A | pages = 303–313 | date = June 2018 | pmid = 29886951 | doi = 10.1016/j.marpolbul.2018.04.029 | s2cid = 47009972 }}</ref> but more research is also needed. There is a need for more research on membrane transport mechanisms, which transfer PFAS into marine organisms, and the biological behavior of shorter chain PFASs.<ref>{{cite journal | vauthors = Sun JM, Kelly BC, Gobas FA, Sunderland EM | title = A food web bioaccumulation model for the accumulation of per- and polyfluoroalkyl substances (PFAS) in fish: how important is renal elimination? | journal = Environmental Science. Processes & Impacts | volume = 24 | issue = 8 | pages = 1152–1164 | date = August 2022 | pmid = 35678632 | doi = 10.1039/D2EM00047D | pmc = 9384792 }}</ref> |
Bioaccumulation and biomagnification of PFASs in marine species throughout the food web, particularly frequently consumed fish and shellfish, can have important impacts on human populations.<ref>{{cite journal | vauthors = Choi S, Kim JJ, Kim MH, Joo YS, Chung MS, Kho Y, Lee KW | title = Origin and organ-specific bioaccumulation pattern of perfluorinated alkyl substances in crabs | journal = Environmental Pollution | volume = 261 | pages = 114185 | date = June 2020 | pmid = 32114125 | doi = 10.1016/j.envpol.2020.114185 | s2cid = 211727091 }}</ref> PFASs have been frequently documented in both fish and shellfish that are commonly consumed by human populations.<ref name="Perfluoroalkyl substances PFASs i">{{cite journal | vauthors = Fair PA, Wolf B, White ND, Arnott SA, Kannan K, Karthikraj R, Vena JE | title = Perfluoroalkyl substances (PFASs) in edible fish species from Charleston Harbor and tributaries, South Carolina, United States: Exposure and risk assessment | journal = Environmental Research | volume = 171 | pages = 266–277 | date = April 2019 | pmid = 30703622 | pmc = 6943835 | doi = 10.1016/j.envres.2019.01.021 | bibcode = 2019ER....171..266F }}</ref> This poses health risks to humans and studies on the bioaccumulation in certain species are important to determine daily tolerable limits for human consumption, and where those limits may be exceeded causing potential health risks.<ref>{{Cite journal | vauthors = Teunen L, Bervoets L, Belpaire C, De Jonge M, Groffen T |date=2021-03-29 |title=PFAS accumulation in indigenous and translocated aquatic organisms from Belgium, with translation to human and ecological health risk |journal=Environmental Sciences Europe |volume=33 |issue=1 |pages=39 |doi=10.1186/s12302-021-00477-z |s2cid=232414650 |issn=2190-4715}}</ref> This has particular implications for populations that consume larger numbers of wild fish and shellfish species.<ref name="Perfluoroalkyl substances PFASs i"/> In addition to health risks, populations may be impacted by advisories, limits of closures of fishing for certain species that are put in place to help mitigate health risks from potential consumption of species with higher levels of accumulated PFASs, but result in a loss of food sources and important subsistence species depended on by local communities. There is much research being done in this area, including into spatial patterns of PFAS bioaccumulation in fish and crustaceans<ref>{{cite journal | vauthors = Taylor MD, Beyer-Robson J, Johnson DD, Knott NA, Bowles KC | title = Bioaccumulation of perfluoroalkyl substances in exploited fish and crustaceans: Spatial trends across two estuarine systems | journal = Marine Pollution Bulletin | volume = 131 | issue = Pt A | pages = 303–313 | date = June 2018 | pmid = 29886951 | doi = 10.1016/j.marpolbul.2018.04.029 | s2cid = 47009972 }}</ref> but more research is also needed. There is a need for more research on membrane transport mechanisms, which transfer PFAS into marine organisms, and the biological behavior of shorter chain PFASs.<ref>{{cite journal | vauthors = Sun JM, Kelly BC, Gobas FA, Sunderland EM | title = A food web bioaccumulation model for the accumulation of per- and polyfluoroalkyl substances (PFAS) in fish: how important is renal elimination? | journal = Environmental Science. Processes & Impacts | volume = 24 | issue = 8 | pages = 1152–1164 | date = August 2022 | pmid = 35678632 | doi = 10.1039/D2EM00047D | pmc = 9384792 }}</ref> |
||
Revision as of 21:14, 5 March 2023
This article may require copy editing for grammar, style, cohesion, tone, or spelling. (April 2022) |
Per- and polyfluoroalkyl substances (PFASs) are synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain. An early definition, from 2011, required that they contain at least one perfluoroalkyl moiety, –CnF2n+1–.[1][2] Beginning in 2021, the Organisation for Economic Co-operation and Development (OECD) expanded their terminology, stating that "PFASs are defined as fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e. with a few noted exceptions, any chemical with at least a perfluorinated methyl group (–CF3) or a perfluorinated methylene group (–CF2–) is a PFAS."[3][4]
According to the OECD, at least 4,730 distinct PFASs are known, which contain at least three perfluorinated carbon atoms.[5] The United States Environmental Protection Agency (EPA) toxicity database, DSSTox, lists 14,735 unique PFAS chemical compounds.[6] PubChem lists approximately 6 million.[7] The fluorosurfactants or fluorinated surfactants subgroup, has a fluorinated "tail" and a hydrophilic "head" and are thus considered surfactants. These are more effective at reducing the surface tension of water than comparable hydrocarbon surfactants. They include the perfluorosulfonic acids, such as perfluorooctanesulfonic acid (PFOS) and the perfluorocarboxylic acids like perfluorooctanoic acid (PFOA).
Many PFASs were used in the mid-20th century in products and on materials due to their enhanced water-resistant properties, such as within Teflon or aqueous film forming foam. Only since the start of the 21st century has the environmental impact and toxicity to human and mammalian life been studied in depth. PFOS, PFOA and other PFASs are commonly described as persistent organic pollutants because they remain in the environment for long periods of time, and are also known as "forever chemicals". Residues have been detected in humans and wildlife, prompting concern about impacts to health.[8][9][10] According to the National Academies of Sciences, Engineering, and Medicine, PFAS exposure is linked to increased risk of dyslipidemia (abnormally high cholesterol), suboptimal antibody response, reduced infant and fetal growth, and higher rates of kidney cancer.[11]
Health concerns related to PFASs have resulted in numerous litigations (see Timeline of events related to per- and polyfluoroalkyl substances). In 2021, Maine became the first U.S. state to ban these compounds in all products by 2030, except for instances deemed "currently unavoidable".[12][13]
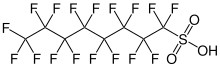

Fluorosurfactants

Fluorosurfactants are surfactants containing fluorocarbon chains such as those in PFASs. They can reduce the surface tension of water below what is attainable by using hydrocarbon surfactants.[14] This ability is due to the hydrophobic nature of fluorocarbons, so fluorosurfactants tend to concentrate at the liquid-air interface.[15] Fluorocarbons are lipophobic, as well as hydrophobic, allowing them to repel both oil and water. This lipophobicity results from the lack of attractive London dispersion forces in fluorocarbons compared to hydrocarbons, a consequence of fluorine's large electronegativity and small bond length, which reduce the polarizability of the surfactants' fluorinated molecular surface. Fluorosurfactants are more stable and fit for harsher conditions than hydrocarbon surfactants because of the stability of the carbon–fluorine bond. Perfluorinated surfactants persist in the environment for this same reason.[8]
Economic role
PFASs play a key economic role for companies such as DuPont, 3M, and W. L. Gore & Associates because they are used in emulsion polymerization to produce fluoropolymers. They have two main markets: a $1 billion annual market for use in stain repellents, and a $100 million annual market for use in polishes, paints, and coatings.[16]
Health and environmental concerns
| Part of a series on |
| Pollution |
|---|
 |
Human health concerns associated with PFASs
On their introduction in the 1940s, per- and polyfluoroalkyl substances (PFASs) were considered inert.[17][18] In fact, early occupational studies revealed elevated levels of fluorochemicals, including perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA, C8), in the blood of exposed industrial workers, but cited no ill health effects.[19][20] These results were consistent with the measured serum concentrations of PFOS and PFOA in 3M plant workers ranging from 0.04 to 10.06 ppm and 0.01 to 12.70 ppm respectively, well below toxic and carcinogenic levels cited in animal studies.[20] Given, however, the "forever chemical" property of PFASs (serum elimination half-life 4–5 years) and widespread environmental contamination, molecules have been shown to accumulate in humans to such a degree that adverse health outcomes have resulted.[17]
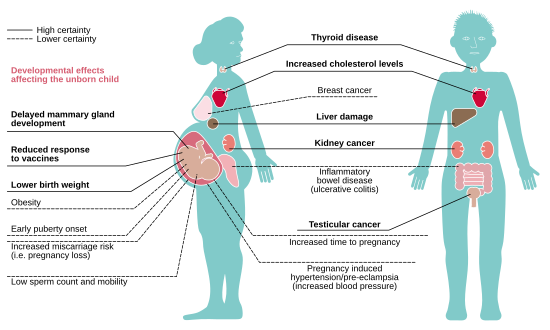
Hormone-disrupting chemicals, including PFASs, are linked with rapid declines in human fertility.[27] In a meta-analysis for associations between PFASs and human clinical biomarkers for liver injury, authors considered both PFAS effects on liver biomarkers and histological data from rodent experimental studies and concluded that evidence exists showing that PFOA, perfluorohexanesulfonic acid (PFHxS), and perfluorononanoic acid (PFNA) are hepatotoxic to humans.[28]
Many comprehensive epidemiological studies linking adverse human health effects to PFASs, particularly PFOA, come from the C8 Science Panel.[29] This panel was formed as part of a contingency to a class action lawsuit brought by communities in the Ohio River Valley against DuPont in response to landfill and wastewater dumping of PFAS-laden material from DuPont's West Virginia Washington Works Plant.[29] The panel measured PFOA (also known as C8) serum concentrations in 69,000 individuals from around DuPont's Washington Works Plant and found a mean concentration of 83.0 ng/mL, compared to 4 ng/mL in a standard population of Americans.[30] This panel reported probable links[vague] between elevated PFOA blood concentration and hypercholesterolemia, ulcerative colitis, thyroid disease, testicular cancer, kidney cancer as well as pregnancy-induced hypertension and preeclampsia.[31][32][33][34][35]
Prevalence in rainwater
In 2022 it was found that levels of at least four perfluoroalkyl acids (PFAAs) in rainwater worldwide ubiquitously, and often greatly, exceeded the EPA's lifetime drinking water health advisories as well as comparable Danish, Dutch, and European Union safety standards, leading researchers to conclude that "the global spread of these four PFAAs in the atmosphere has led to the planetary boundary for chemical pollution being exceeded".[36] There are some moves to restrict and replace their use.[37]
Estimated contemporary costs
In a report by the Nordic Council of Ministers the total annual health-related costs associated with human exposure to PFASs were estimated to be at least €52-€84 billion in the European Economic Area (EEA) countries.[38] For the United States, estimated PFAS-attributable disease costs amount to 6–62 billion US$.[39] Aggregated annual costs covering environmental screening, monitoring where contamination is found, water treatment, soil remediation and health assessment total €821 million-€170 billion in the EEA plus Switzerland.[38]
Hypercholesterolemia
Animal studies in the 1990s and early 2000s primarily aimed to investigate the effect of two widely used long-chain PFASs, perfluorooctanoic acid (PFOA, C8) and perfluorooctane sulphonic acid (PFOS, C8), on peroxisome proliferation in rat livers.[40] These studies determined that PFOA and PFOS acted as peroxisome proliferator-activated receptor (PPAR) agonists, increasing lipid metabolism.[40] A paradoxical response is observed in humans where elevated PFOS levels were significantly associated with[vague] elevated total cholesterol and LDL cholesterol, highlighting significantly reduced PPAR expression and alluding to PPAR independent pathways predominating over lipid metabolism in humans compared to rodents.[41]
Ulcerative colitis
PFOA and PFOS have been shown to significantly alter immune and inflammatory responses in human and animal species. In particular, IgA, IgE (in females only) and C-reactive protein have been shown to decrease whereas antinuclear antibodies increase as PFOA serum concentrations increase.[42] These cytokine variations allude to immune response aberrations resulting in autoimmunity. One proposed mechanism is a shift towards anti-inflammatory M2 macrophages and/or T-helper (TH2) response in intestinal epithelial tissue which allows sulfate-reducing bacteria to flourish. Elevated levels of hydrogen sulfide result which reduce beta-oxidation and thus nutrient production leading to a breakdown of the colonic epithelial barrier.[43]
Thyroid disease
Hypothyroidism is the most common thyroid abnormality associated with[vague] PFAS exposure.[44] PFASs have been shown to decrease thyroid peroxidase, resulting in decreased production and activation of thyroid hormones in vivo.[45] Other proposed mechanisms include alterations in thyroid hormone signaling, metabolism and excretion as well as function of nuclear hormone receptor.[44]
Cancer
Rat studies investigating the carcinogenicity of PFASs reported significant correlation with liver adenomas, Leydig cell tumors of the testis and pancreatic acinar cell tumors and dietary PFOA consumption.[45] The C8 Science Panel investigated the potential relationship between PFAS exposure and these three cancer types as well as 18 other cancer types in their epidemiological studies. Contrary to the animal studies, the C8 studies did not find a probable link[vague] between elevated C8 exposure and liver adenomas or pancreatic acinar cell tumors; however, a probable link[vague] was found with regards to testis and kidney cancer.[46] Two mechanisms have been proposed by which PFOA could cause Leydig cell tumors. Both mechanisms start by proposing that PROA exposure results in increased PPAR alpha activation in the liver which increases hepatic aromatase concentration and subsequent serum estrogen levels. The mechanisms now diverge, with one pathway suggesting elevated estradiol levels increase Tissue Growth Factor alpha (TGF alpha) which prompts Leydig cell proliferation. The other pathway suggests that aromatization of testosterone to estradiol reduces serum testosterone levels resulting in increased release of luteinizing hormone (LH) from the pituitary gland which directly results in Leydig Cell tumorgenesis.[47] A mechanism has not yet been proposed to explain how kidney cancer could be caused by C8 exposure as no in vivo animal studies have been able to model this epidemiological outcome.[48]
Pregnancy-induced hypertension and pre-eclampsia
Pregnancy-induced hypertension is diagnosed when maternal systolic blood pressure exceeds 140 mmHg or diastolic blood pressure exceeds 90mmHg after 20 weeks gestation.[49] Diagnostic criteria are the same for pre-eclampsia as pregnancy-induced hypertension; however, it also confers proteinuria. Mechanisms by which pregnancy-induced hypertension and preeclampsia could be caused by PFAS exposure have remained elusive and are largely speculative to date. One proposed mechanism highlights alterations in immune function leading to disruption of placentation, specifically as it pertains to natural killer (NK) cell infiltration of the placenta to facilitate trophoblastic integration with placental blood supply.[50] Another mechanism refers to agonism of PPARs contributing to alterations in cholesterol, triglyceride and uric acid levels which may lead to vascular inflammation and elevated blood pressure.[50]
Other adverse health outcomes that have been attributed to elevated PFAS exposure but were not found to be probable links[vague] in the C8 studies are decreased antibody response to vaccines, asthma, decreased mammary gland development, low birth weight (-0.7oz per 1 ng/mL increase in blood PFOA or PFOS level), decreased bone mineral density and neurodevelopmental abnormalities.[51][52][53]
"Forever chemicals"
Fluorosurfactants such as PFOS, PFOA, and PFNA have caught the attention of regulatory agencies because of their persistence, toxicity, and widespread occurrence in the blood of general populations[54][55] and wildlife. In 2009, PFOS, its salts and perfluorooctanesulfonyl fluoride were listed as persistent organic pollutants under the Stockholm Convention, due to their ubiquitous, persistent, bioaccumulative, and toxic nature.[56][57] PFAS chemicals were dubbed the "Forever Chemicals" following a 2018 op-ed in the Washington Post.[58] The nickname was derived by combining the two dominant attributes of this class of chemicals: 1) PFAS chemicals are characterized by a carbon-fluorine (C-F) backbone (the "F-C" in "Forever Chemicals"); and 2) the carbon fluorine bond is one of the strongest bonds in organic chemistry, which gives these chemicals an extremely long environmental half-life (the "Forever" in "Forever Chemicals"). The Forever Chemicals name is now commonly used in media outlets in addition to the more technical name of per- and polyfluorinated alkyl substances, or PFASs.[59][60][61][62] Their production has been regulated or phased out by manufacturers, such as 3M, DuPont, Daikin, and Miteni in the US, Japan, and Europe. In 2006 3M replaced PFOS and PFOA with short-chain PFASs,[16] such as perfluorohexanoic acid (PFHxA) and perfluorobutanesulfonic acid (PFBS). Shorter fluorosurfactants may be less prone to accumulating in mammals;[16] there is still concern that they may be harmful to both humans,[63][64][65] and the environment.[66] A majority of PFASs are either not covered by European legislation or are excluded from registration obligations under the EU REACH chemical regulation.[67] Several PFASs have been detected in drinking water,[68] municipal wastewater[69] and landfill leachates,[70] worldwide.
It had been thought that perfluoroalkyl acids (PFAAs) would eventually end up in the oceans, where they would be diluted over decades, but a field study published in 2021 by researchers at Stockholm University found that they are significantly transferred from water to air when waves break on land, and are a significant source of air pollution, and eventually get into the rain. The researchers concluded that pollution "may impact large areas of inland Europe and other continents, in addition to coastal areas".[71][72]
Bioaccumulation and biomagnification

Bioaccumulation is the process by which PFASs are transferred into the tissue of any exposed organisms where PFASs accumulate over time since organisms lack natural excretion mechanisms. PFASs can accumulate in marine species by a variety of pathways. They can be absorbed from the environment, such as contaminated sediments or PFASs dissolved in water. PFASs can partition into the organs and tissues of marine organisms from these environmental compartments. They have been shown to bind to blood proteins and accumulate in the livers of marine animals.[73] Another pathway for bioaccumulation is predation. As larger marine animals feed on smaller organisms that have been exposed to PFASs, the larger animals absorb the PFASs contained in their prey.

Biomagnification is the process by which the amount of PFAS contamination increases with increasing trophic level, due to predation by the species higher in the food web. Top predators have higher levels of PFASs than species lower down the food chain. Seabirds that feed on fish have among the highest levels of PFAS contamination.[73] Perfluorosulfonic acids, which have a sulfonic acid functional group attached to the fluorinated "tail", have a greater tendency to bioaccumulate than perfluorocarboxylic acids, which contain a carboxylic acid function group. Longer chain PFAS compounds, which have 6, 7, or more fluorinated carbons, bioaccumulate in greater quantities than shorter chain PFAS compounds.
- In marine species of the food web
The concentration of PFOS, a long chain sulfonic acid, was found at the highest concentrations relative to other PFASs measured in fish and birds in Northern seas such as the Barents Sea and the Canadian Arctic.[74] A study and an interactive map by EWG using its results showed freshwater fish in the U.S.[globalize] ubiquitously contain high levels of harmful PFAS, with a single serving typically significantly increasing the blood PFOS level.[75][76]
Bioaccumulation and biomagnification of PFASs in marine species throughout the food web, particularly frequently consumed fish and shellfish, can have important impacts on human populations.[77] PFASs have been frequently documented in both fish and shellfish that are commonly consumed by human populations.[78] This poses health risks to humans and studies on the bioaccumulation in certain species are important to determine daily tolerable limits for human consumption, and where those limits may be exceeded causing potential health risks.[79] This has particular implications for populations that consume larger numbers of wild fish and shellfish species.[78] In addition to health risks, populations may be impacted by advisories, limits of closures of fishing for certain species that are put in place to help mitigate health risks from potential consumption of species with higher levels of accumulated PFASs, but result in a loss of food sources and important subsistence species depended on by local communities. There is much research being done in this area, including into spatial patterns of PFAS bioaccumulation in fish and crustaceans[80] but more research is also needed. There is a need for more research on membrane transport mechanisms, which transfer PFAS into marine organisms, and the biological behavior of shorter chain PFASs.[81]
Australia
In 2017, the ABC's current affairs programme Four Corners reported that the storage and use of firefighting foams containing perfluorinated surfactants at Australian Defence Force facilities around Australia had contaminated nearby water resources.[82] In 2019, remediation efforts at RAAF Base Tindal and the adjacent town of Katherine were ongoing.[83] In the 2022 Australian federal budget $428 million was allocated for works at HMAS Albatross, RAAF Base Amberley, RAAF Base Pearce and RAAF Base Richmond including funding to remediate PFAS contamination.[84]
Canada
Although PFASs are not manufactured in Canada, they may be present in imported goods and products. In 2008, Canada prohibited the import, sale, or use of PFOS or PFOS-containing products, with some exceptions for products used in firefighting, in the military, and some forms of ink and photo media.[85]
Health Canada has published drinking water guidelines for maximum concentrations of PFOS and PFOA. The guidelines were established to protect the health of Canadians, including children, over a lifetime's exposure to these substances. The maximum allowable concentration for PFOS under the guidelines is 0.0002 milligrams per litre. The maximum allowable concentration for PFOA is 0.0006 milligrams per litre.[86]
United Kingdom
The environmental consequences of PFAS - especially from fire fighting activities - has been recognised since the mid 1990s and came to prominence after the Buncefield explosion on 11 December 2005. In recent years the Environment Agency has undertaken a series of projects to understand the scale and nature of PFAS in the environment. The Drinking Water Inspectorate requires water companies to report concentrations of 47 PFAS.Requirements for PFAS monitoring by water companies in England and Wales
European Union
In 2019, the European Council requested the European Commission to develop an action plan to eliminate all non-essential uses of PFAS due to the growing evidence of adverse effects caused by exposure to these substances, the evidence for the widespread occurrence of PFAS in water, soil, articles and waste and the threat it can pose to drinking water.[87] At the initiative of Germany and the Netherlands, these countries, together with Denmark, Norway and Sweden submitted a so-called restriction proposal based on the REACH Regulation to achieve a European ban on the production, use, sale and import of PFAS.[88] The proposal states that a ban is necessary for all use of PFAS, with different periods for different applications when the ban takes effect (immediately after the restriction comes into force, 5 years afterwards or 12 years afterwards), depending on the function and the availability of alternatives. The proposal has not assessed the use of PFAS in medicines, plant protection products and biocides because specific regulations apply to those substances (Biocidal Products Regulation, Plant Protection Products Regulation, Medicinal Products Regulation) that have an explicit authorization procedure that focuses on risk for health and the environment.
The proposal was submitted on 13 January 2023 and published by the European Chemicals Agency (ECHA) on 7 February of the same year. From 22 March to 21 September 2023, citizens, companies and other organizations can comment on the proposal during a public consultation.[89] Based on the information in the restriction proposal and the consultation, two committees from ECHA formulate an opinion on the risk and socio-economic aspects of the proposed restriction, respectively. Within a year of publication, the opinions are sent to the European Commission, which then makes a final proposal that is submitted to the EU Member States for discussion and decision.[90] Eighteen months after the publication of the restriction decision, it will enter the ban. Of course, the restriction may differ from the proposal.[89]
Italy
Over 350,000 residents in Veneto are estimated to have been exposed to contamination through tap water, it is Europe's biggest PFAS-related environmental disaster. While Italy's National Health Institute (ISS, Istituto Superiore di Sanità) set the threshold limit of PFOA in the bloodstream at 8 nanograms per milliliter (ng/mL), some residents had reached 262 and some industrial employees reach 91,900 ng/mL. In 2021 some data has been disclosed by Greenpeace and other local citizens after a long legal battle against the Veneto Region and ISS, which for years has denied access to data that despite the alarming values known since or even before 2017 the Veneto Region has not carried out further monitoring or taken resolutive actions to eliminate pollution and reduce, at least gradually, the contamination of water not intended for drinking. Furthermore, as far as is known, it appears that the Region has so far ignored the risk for the entire national community and beyond, given that some of these foods could also be sold abroad. Although in 2020 the European Food Safety Agency (EFSA) has reduced by more than four times the maximum tolerable limit of PSAS that can be taken through the diet, the Region has not carried out new assessments or implemented concrete actions to protect the population and the agri-food and livestock sectors. To this are added some limits on the monitoring of the monitored geographical area, which does not include the orange zone and other areas affected by contamination, as well as the insufficiency of analysis on important productions widespread in the areas concerned: eggs (up to 37100 ng/kg), fish (18600 ng/kg) spinach and radicchio (only one sampling carried out), kiwis, melons, watermelons, cereals (only one spelled sample was analyzed), soy, wines (very famous from the region) and apples.[91] The most polluted area is near the Lombardia region which has no data public for this kind of pollutant.[citation needed]
United States
In products

Certain PFASs are no longer manufactured in the United States, as a result of phase-outs including the PFOA Stewardship Program (2010-2015), in which eight major chemical manufacturers agreed to eliminate the use of PFOA and PFOA-related chemicals in their products and as emissions from their facilities.[92] Although PFOA and PFOS are no longer manufactured in the United States, they are still produced internationally and are imported into the US in consumer goods such as carpet, leather and apparel, textiles, paper and packaging, coatings, rubber and plastics.[93]
In 2020, manufacturers and the Food and Drug Administration (FDA) announced an agreement to phase out some types of PFAS which are used in food packaging by 2024.[94]
PFASs are also used by major companies of the cosmetics industry in a wide range of cosmetics, including lipstick, eye liner, mascara, foundation, concealer, lip balm, blush, nail polish and other such products. A 2021 study tested 231 makeup and personal care products and found organic fluorine, an indicator of PFASs, in more than half of the samples. High levels of fluorine were most commonly identified in waterproof mascara (82% of brands tested), foundations (63%), and liquid lipstick (62%).[95][96] As many as 13 types of individual PFAS compounds were found in each product.[95] Since PFAS compounds are highly mobile, they are readily absorbed through human skin and through tear ducts, and such products on lips are often unwittingly ingested. Manufacturers often fail to label their products as containing PFASs, which makes it difficult for cosmetics consumers to avoid products containing PFASs.[97] In response, Senators Susan Collins of Maine and Richard Blumenthal of Connecticut proposed the No PFAS in Cosmetics Act in the United States Senate.[98] It was also introduced in the United States House of Representatives by Michigan Representative Debbie Dingell.[99] The U.S. chemical industry lobby however, armed with many billions of dollars, has killed efforts to regulate this.[100]
Contaminated sites, drinking water and wastewater
An estimated 26,000 U.S. sites are contaminated with PFASs. At least six million Americans are estimated to have drinking water containing PFASs above the safe limit published prior to 2022 by the U.S. Environmental Protection Agency (EPA).[101][102] More than 200 million Americans are estimated to live in places where the tap water PFAS level (a combination of PFOA and PFOS levels) exceeds the 1 ppm (part per million) limit set in 2022 by the EPA.[103]
EPA published non-enforceable drinking water health advisories for PFOA and PFOS in 2016.[104][105] In March 2021 EPA announced that it will develop national drinking water standards for PFOA and PFOS.[106] On December 27, 2021, EPA published a regulation requiring drinking water utilities to conduct monitoring for 29 compounds. The data are to be collected during 2023 to 2025. EPA will pay for the monitoring costs for small drinking water systems (those serving a population of 10,000 or fewer). The agency may use the monitoring data to develop additional regulations.[107][108]
In mid-2021 EPA announced plans to revise federal wastewater regulations (effluent guidelines) for several industries that manufacture PFASs or use PFASs in fabricating various products.[109][110]
In October 2021 EPA announced the PFAS Strategic Roadmap. This initiative is a "whole-of-EPA" strategy and considers the full lifecycle of PFAS—including drinking water monitoring and risk assessment for PFOA and PFOS in biosolids (processed wastewater sludge used as fertilizer).[111][112]
The EPA issued health advisories for four specific PFASs in June 2022, significantly lowering their safe threshold levels for drinking water. PFOA was reduced from 70 ppt to 0.004 ppt, while PFOS was reduced from 70 ppt to 0.02 ppt. GenX's safe levels were set at 10 ppt, while PFBS were set to 2000 ppt. While not enforceable, these health advisories are intended to be acted on by states in setting their own drinking water standards.[113]
A formal EPA rule to add PFOA and PFAS as hazardous chemicals was first issued for comment on August 26, 2022. This would require those discharging waste to monitor and restrict the release of these PFAS to set levels, and report when the wastewater exceeds it. It would also make grounds affected by high levels of PFIA or PFAS to be treated as Superfund cleanup sites.[114]
EPA has listed recommended steps that consumers may take to reduce possible exposure to PFAS chemicals.[115]
California
In 2021 California banned PFASs for use in food packaging and from infant and children's products and also required PFAS cookware in the state to carry a warning label.[116]
Maine
A program licensed and promoted by the Maine Department of Environmental Protection that provided free municipal wastewater sludge (biosolids) to farmers as fertilizer has resulted in PFAS contamination of local drinking water and farm-grown produce.[117][118]
Michigan
Launched in 2017, the Michigan PFAS Action Response Team (MPART) is the first multi-agency action team of its kind in the nation. Agencies representing health, environment, and other branches of state government have joined together to investigate sources and locations of PFAS contamination in the state, take action to protect people's drinking water, and keep the public informed.[119]
Groundwater is tested at locations throughout the state by various parties to ensure safety, compliance with regulations, and proactively detect and remedy potential problems. In 2010, the Michigan Department of Environmental Quality (MDEQ) discovered levels of PFASs in groundwater monitoring wells at the former Wurtsmith Air Force Base. As additional information became available from other national testing, Michigan expanded its investigations into other locations where PFAS compounds were potentially used.[119]
In 2018, the MDEQ's Remediation and Redevelopment Division (RRD) established cleanup criteria for groundwater used as drinking water of 70 ppt of PFOA and PFOS, individually or combined. The RRD staff are responsible for implementing these criteria as part of their ongoing efforts to clean up sites of environmental contamination. The RRD staff are the lead investigators at most of the PFAS sites on the MPART website and also conduct interim response activities, such as coordinating bottled water or filter installations with local health departments at sites under investigation or with known PFAS concerns. Most of the groundwater sampling at PFAS sites under RRD's lead is conducted by contractors familiar with PFAS sampling techniques. The RRD also has a Geologic Services Unit, with staff who install monitoring wells and are also well versed with PFAS sampling techniques.[119]
The MDEQ has been conducting environmental clean-up of regulated contaminants for decades. Due to the evolving nature of PFAS regulations as new science becomes available, the RRD is evaluating the need for regular PFAS sampling at Superfund sites and is including an evaluation of PFAS sampling needs as part of a Baseline Environmental Assessment review.[119]
Earlier in 2018, the RRD purchased lab equipment that will allow the MDEQ Environmental Lab to conduct analyses of certain PFAS samples. (Currently, most samples are shipped to one of the few labs in the country that conduct PFAS analysis, in California, although private labs in other parts of the country, including Michigan, are starting to offer these services.) As of August 2018, RRD has hired additional staff to work on developing the methodology and conducting PFAS analyses.[119]
In 2020 Michigan Attorney General Dana Nessel filed a lawsuit against 17 companies, including 3M, Chemours, and DuPont, for hiding known health and environmental risks from the state and its residents. Nessel's complaint identifies 37 sites with known contamination.[120]
In 2020 the Michigan Department of Environment, Great Lakes, and Energy (EGLE) introduced some of the strictest drinking water standards in the country for PFAS, setting maximum contaminant levels (MCLs) for PFOA and PFOS to 8 and 16 ppt respectively (down from previous existing groundwater cleanup standards of 70 ppt for both), and introducing MCLs for 5 other previously unregulated PFAS compounds, limiting PFNA to 6 ppt, PFHxA to 400,000 ppt, PFHxS to 51 ppt, PFBS to 420 ppt and HFPO-DA to 370 ppt.[121] The change adds 38 additional sites to the state's list of known PFAS contaminated areas, bringing the total number of known sites to 137. About half of these sites are landfills and 13 are former plating facilities.[122]
In 2022 PFOS was found in beef produced at a Michigan farm. The cattle had been fed crops fertilized with contaminated biosolids. State agencies issued a consumption advisory, but did not order a recall, because there currently is no PFOS contamination in beef government standards.[123]
Minnesota
In February 2018, 3M settled a lawsuit for $850 million related to contaminated drinking water in Minnesota.[124]
New Jersey
In 2018 the New Jersey Department of Environmental Protection (NJDEP) published a drinking water standard for PFNA. Public water systems in New Jersey are required to meet a maximum contaminant level (MCL) standard of 13 ppt.[125][126] In 2020 the state set a PFOA standard at 14 ppt and a PFOS standard at 13 ppt.[127]
In 2019 NJDEP filed lawsuits against the owners of two plants that had manufactured PFASs, and two plants that were cited for water pollution from other chemicals. The companies cited are DuPont, Chemours and 3M.[128] NJDEP also declared five companies to be financially responsible for statewide remediation of the chemicals. Among the companies accused were Arkema and Solvay regarding a West Deptford Facility in Gloucester County, where Arkema manufactured PFASs, but Solvay claims to have never manufactured but only handled PFASs.[129] The companies denied liability and contested the directive.[130] In June 2020, the US Environmental Protection Agency and NJ Department of Environmental Protection published a paper reporting that a unique family of PFAS used by Solvay, chloroperfluoropolyether carboxylates (ClPFPECAs), were contaminating the soils of New Jersey as far from the Solvay facility as 150 km.[131] and the ClPFPECAs were found in water as well.[132]
Later in 2020, the New Jersey state attorney general filed suit in the New Jersey Superior Court against Solvey regarding PFAS contamination of the state's environment.[133] In May 2021, Solvay issued a press release that the company is "discontinuing the use of fluorosurfactants in the U.S.".[134]
Washington
Washington State has a history of PFAS releases to the environment.[135][136][137]
In addition, five military installations in Washington State have been identified by the U.S. Senate Committee on Environment and Public Works as having PFAS contamination. Toward environmental and consumer protections, the Washington State Department of Ecology published a Chemical Action Plan in November 2021, and in June 2022 the governor tasked the Washington State Department of Ecology with phasing out manufacture and import of products containing PFASs. Initial steps taken by the Washington State Department of Health to protect the public from exposure through drinking water have included setting State Action Levels for five PFASs (PFOA, PFOS, PFNA, PFHxS, and PFBS), which were implemented in November 2021.
Class action lawsuits
In February 2017, DuPont and Chemours (a DuPont spin-off) agreed to pay $671 million to settle lawsuits arising from 3,550 personal injury claims related to releasing of PFASs from their Parkersburg, West Virginia plant, into the drinking water of several thousand residents.[138] This was after a court-created independent scientific panel, the "C8 Science Panel", found a "probable link" between C8 exposure and six illnesses: kidney and testicular cancer, ulcerative colitis, thyroid disease, pregnancy-induced hypertension and high cholesterol.[29][139]
In October 2018, a class action suit was filed by an Ohio firefighter against several producers of fluorosurfactants, including the 3M and DuPont corporations, on behalf of all US residents who may have adverse health effects from exposure to PFASs.[140]
This story is told in the film Dark Waters, released in November 2019, produced by the actor Mark Ruffalo and directed by Todd Haynes.[141]
Corporate and federal government suppression of information
Starting in the 1970s, 3M scientists learned that PFOS and PFOA were toxic to humans, documenting damage to the human immune system. Also in the 1970s, 3M scientists found that these substances accumulate over time in the human body. However, 3M suppressed revelation of these facts to the public or to regulators.[142]
In 2018 White House staff and EPA pressured the U.S. Agency for Toxic Substances and Disease Registry to suppress a study that showed PFASs to be even more dangerous than previously thought.[143][144]
Water contamination by U.S. military bases
The water in and around at least 126 U.S. military bases has been contaminated by high levels of PFASs because of their use of firefighting foams since the 1970s, according to a study by the U.S. Department of Defense. Of these, 90 bases reported PFAS contamination that had spread to drinking water or groundwater off the base.[145][146] A 2022 Pentagon report acknowledged that approximately 175,000 U.S. military personnel at two dozen American military facilities drank water contaminated by higher PFAS levels than the U.S. Environmental Protection Agency limit. However, according to an analysis of the Pentagon report by the non-partisan Environmental Working Group, the Pentagon report downplayed the number of people exposed to PFAS, which was much higher, probably in excess of 640,000 at 116 military facilities, than the number advanced by the Pentagon report. The EWG found that the Pentagon also omitted from its report some types of diseases that are likely to be caused by PFAS exposure, omitting testicular cancer, kidney disease, and fetal abnormalities.[147]
Occupational exposure
Occupational exposure to PFASs occurs in numerous industries due to the widespread use of PFASs in products and as an element of industrial process streams.[22] PFASs are used in more than 200 different ways in industries as diverse as electronics and equipment manufacturing, plastic and rubber production, food and textile production, and building and construction.[148] Occupational exposure to PFASs can occur at fluorochemical facilities that produce PFASs and other manufacturing facilities that use PFASs for industrial processing like the chrome plating industry.[22] Workers who handle PFAS-containing products can also be exposed during their work. Examples include people who install PFAS-containing carpets and leather furniture with PFAS coatings, professional ski-waxers using PFAS-based waxes, and fire-fighters who use PFAS-containing foam and wear flame-resistant protective gear impregnated with PFASs.[22][149][150][151][152]
People who are exposed to PFASs through their jobs typically have higher levels of PFASs in their blood than the general population.[22][150][151][153][154] Additionally, while the general population is exposed to PFASs through ingested food and water, occupational exposure includes both accidental ingestion and inhalation exposure in settings where a PFAS becomes volatilized.[155] There has been increased attention to the health risks associated with exposure to PFASs, which can affect the immune system, increase cholesterol, and increase the risk of cancer.[51] The severity of PFAS-associated health effects can vary based on the length of exposure, level of exposure, and health status.[22] In 2009, under decision SC-4/17, perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride were listed in Annex B of the 2009 Stockholm Convention on Persistent Organic Pollutants, dictating acceptable purposes and specific exemptions to the chemical usage. Among these exemptions are numerous uses in manufacturing as well as firefighting foams.
Professional ski wax technicians
Professional ski wax technicians are disproportionately exposed to PFASs from the glide wax used to coat the bottom of skis to reduce the friction between the skis and snow. During this process, the wax is heated to 130-220 °C, which releases fumes and airborne fluorinated compounds. Exposure to aerosolized PFASs is associated with alveolic edema, polymer fume fever, severe dyspnea, decreased pulmonary function, and respiratory distress syndrome in those chronically exposed.[150] In a 2010 study, blood serum levels of PFOA were significantly higher in ski wax technicians compared to levels of the general Swedish population. Serum levels of PFOA in ski wax technicians were positively correlated with years spent working, suggesting bioaccumulation of PFOA over time.[150]
Manufacturing workers
People who work at fluorochemical production plants and in manufacturing industries that use PFASs in the industrial process can be exposed to PFASs in the workplace. Much of what we know about PFASs exposure and health effects began with medical surveillance studies of workers exposed to PFASs at fluorochemical production facilities. These studies began in the 1940s and were conducted primarily at U.S. and European manufacturing sites. Between the 1940s and 2000s, thousands of workers exposed to PFASs participated in research studies that advanced scientific understanding of exposure pathways, toxicokinetic properties, and adverse health effects associated with exposure.[19][156][157][20][158][159][160][161][162][163][164][165][166]
The first research study to report elevated organic fluorine levels in the blood of fluorochemical workers was published in 1980.[19] This study established inhalation as a potential route of occupational PFAS exposure by reporting measurable levels of organic fluorine in air samples at the facility.[19] Workers at fluorochemical production facilities have higher levels of PFOA and PFOS in their blood than the general population. Serum PFOA levels in fluorochemical workers are generally below 20,000 ng/mL but have been reported as high as 100,000 ng/mL whereas the mean PFOA concentration among non-occupationally exposed cohorts in the same time frame was 4.9 ng/mL.[163][20][167][168][169][170][171][172][173][174] Among fluorochemical workers, those with direct contact with PFASs have higher PFAS concentrations in their blood than those with intermittent contact and those with no direct PFAS contact.[19][156][163] Further, blood PFAS levels decline when direct contact ceases.[163][158][160] Levels of PFOA and PFOS have declined in US and European fluorochemical workers due to improved facilities, increased usage of personal protective equipment, and the phase out of these chemicals from production.[156][164][166] However, occupational exposure to PFASs in manufacturing continues to be an active area of study in China with numerous investigations linking worker exposure to various PFASs.[175][176][177][178][179]
Firefighters

PFASs are commonly used in Class B firefighting foams due to their hydrophobic and lipophobic properties as well as the stability of the chemicals when exposed to high heat.[180] Due to firefighters' potential for exposure to PFASs through these aqueous film forming foams (AFFF), studies raise concerns that firefighters are especially prone to high concentrations of serum PFASs.
Research into occupational exposure for firefighters is emergent, though frequently limited by underpowered study designs. A 2011 cross-sectional analysis of the C8 Health Studies found higher levels of PFHxS in firefighters compared to the sample group of the region, with other PFASs at elevated levels, without reaching statistical significance.[181] A 2014 study in Finland studying eight firefighters over three training sessions observed select PFASs (PFHxS and PFNA) increase in blood samples following each training event.[180] Due to this small sample size, a test of significance was not conducted. A 2015 cross-sectional study conducted in Australia found that accumulation of PFOS and PFHxS was positively associated with years of occupational AFFF exposure through firefighting.[151]
Due to their use in training and testing, recent studies indicate occupational risk for military members and firefighters, as higher levels of PFASs in exposure were indicated in military members and firefighters when compared to the general population.[182] Further, exposure to PFASs is prevalent among firefighters not only due to its use in emergencies but because it is also used in personal protective equipment. In support of these findings, states like Washington and Colorado have moved to restrict and penalize the use of Class B firefighting foam which contains PFASs for firefighter training and testing.[183][184]
Exposure after World Trade Center terrorist attacks
The September 11, 2001 collapse of the World Trade Center buildings in New York City resulted in the release of chemicals from the destruction of construction and electrical material and long-term chemical fires. This collapse caused the release of several toxic chemicals, including fluorinated surfactants used as soil- and stain-resistant coatings on various materials.[185] First responders to this incident were exposed to PFOA, PFNA, and PFHxS, through inhalation of dust and smoke released during and after the collapse of the World Trade Center.[185]
Fire responders who were working at or near ground zero were assessed for respiratory and other health effects from exposure to emissions at the World Trade Center. Early clinical testing showed a high prevalence of respiratory health effects. Early symptoms of exposure often presented with persistent coughing and wheezing. PFOA and PFHxS levels were present in both smoke and dust exposure. Yet, first responders with smoke exposures had higher concentrations of PFOA and PFHxS than those with dust exposures.[185]
Remediation solutions
Water treatment
Several technologies are currently available for remediating PFASs in liquids. These technologies can be applied to drinking water supplies, groundwater, industrial wastewater, surface water, and other miscellaneous applications (such as landfill leachate). Influent concentrations of PFASs can vary by orders of magnitude for specific media or applications. These influent values, along with other general water quality parameters (for example, pH) can influence the performance and operating costs of the treatment technologies. The technologies are:
- Sorption
- Granular activated carbon
- Biochar
- Ion exchange
- Precipitation/flocculation/coagulation
- Redox manipulation (chemical oxidation and reduction technologies)
- Membrane filtration
- Reverse osmosis
- Nanofiltration[186]
- Supercritical water oxidation[187]
Private and public sector applications of one or more of these methodologies above are being applied to remediation sites throughout the United States and other international locations.[188] Most solutions involve on-site treatment systems, while others are leveraging off-site infrastructure and facilities, such as a centralized waste treatment facility, to treat and dispose of the PFAS pool of compounds.
Most recently, a 2022 study published in the Journal of Environmental Engineering found that a heat-and pressure-based technique known as supercritical water oxidation destroyed 99% of the PFASs present in a water sample. During this process, oxidizing substances are added to PFAS-contaminated water and then the liquid is heated above its critical temperature of 374 degrees Celsius at a pressure of more than 220 bars. The water becomes supercritical (being neither gas nor liquid), and, in this state, water-repellent substances such as PFASs dissolve much more readily.[187]
Theoretical and early-stage solutions
The Michigan State University-Fraunhofer team has a viable solution to treat PFAS-contaminated wastewater that, in 2018, was reported to be ready for a pilot-scale investigation. The electrochemical oxidation system used boron-doped diamond electrodes, in a process breaking down the contaminants' formidable molecular bonds and cleaning the water while systematically destroying the hazardous compounds.[189]
"EO, or electrochemical oxidation, is a simple, clean, and effective method for destruction of PFASs and other co-contaminants as a complementary procedure to other wastewater treatment processes," said Cory Rusinek, an electrochemist at MSU-Fraunhofer. "If we can remove it from wastewater, we can reduce its occurrence in surface waters."[189]
In September 2019, it was reported Acidimicrobium sp. strain A6 could be a potential remediator of PFAS, including saturated ones such as PFOS.[190] PFAS with unsaturated bonds are easier to break down: the commercial dechlorination culture KB1 (contains Dehalococcoides) is capable of breaking down such substances, but not saturated PFAS. When alternative, easier-to-digest substrates are present, microbes may prefer them over PFAS.[191]
Chemical treatment
A study published in the journal Science in August 2022 indicated that perfluoroalkyl carboxylic acids (PFCAs) are able to be "mineralized" via heating in a polar aprotic solvent such as dimethyl sulfoxide. The study reported that heating PFCAs in an 8 to 1 mixture of dimethyl sulfoxide and water at 80–120 °C (176–248 °F) in the presence of sodium hydroxide, caused the removal of the carboxylic acid group at the end of the carbon chain, creating a perfluoroanion. The perfluoroanion then "mineralizes" into sodium fluoride and other salts such as sodium trifluoroacetate, formate, carbonate, oxalate and glycolate. The process does not work on perfluorosulfonic acids such as PFOS.[192] A more recent study published in Chemical Science shows breakdown of C-F bonds and their mineralization as YF3 or YF6 clusters.[193] Another study in the Journal of the American Chemical Society described the PFAs breakdown using metal-organic frameworks (MOFs).[194]
Example chemicals
Some common per- and polyfluoroalkyl substances:[195][196]
- Polytetrafluoroethylene aka PTFE aka Teflon
- Perfluorinated carboxylic acids
- Fluorotelomers
- Perfluorosulfonic acids
- Others:
| Name | Abbreviation | Structural formula | Molecular weight (g/mol) | CAS No. |
|---|---|---|---|---|
| Perfluorobutane sulfonamide | H-FBSA | C4F9SO2NH2 | 299.12 | 30334-69-1 |
| Perfluoropentanesulfonamide | PFPSA | C5F11SO2NH2 | 349.12 | 82765-76-2 |
| Perfluorohexanesulfonamide | PFHxSA | C6F13SO2NH2 | 399.13 | 41997-13-1 |
| Perfluoroheptanesulfonamide | PFHpSA | C7F15SO2NH2 | 449.14 | 82765-77-3 |
| Perfluorooctanesulfonamide | PFOSA | C8F17SO2NH2 | 499.14 | 754-91-6 |
| Perfluorobutanesulfonyl fluoride | PFBSF | C4F9SO2F | 302.09 | 375-72-4 |
| Perfluorooctanesulfonyl fluoride | PFOSF | C8F17SO2F | 502.12 | 307-35-7 |
Films
- The Devil We Know (2018)
- Dark Waters (2019)
See also
- Timeline of events related to per- and polyfluoroalkyl substances
- Entegris, formerly Fluoroware, of Chaska, MN, manufacturer of teflon components for health and semiconductor Fabs
- FSI International, now TEL FSI
- Polytetrafluoroethylene (PTFE)
- Fluoropolymer, another class of per- and polyfluoroalkyl substances
References
- ^ Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. (October 2011). "Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins". Integrated Environmental Assessment and Management. 7 (4): 513–41. doi:10.1002/ieam.258. PMC 3214619. PMID 21793199.
- ^ Ritscher A, Wang Z, Scheringer M, Boucher JM, Ahrens L, Berger U, et al. (August 2018). "Zürich Statement on Future Actions on Per- and Polyfluoroalkyl Substances (PFASs)". Environmental Health Perspectives. 126 (8): 84502. doi:10.1289/EHP4158. PMC 6375385. PMID 30235423.
- ^ OECD (2021). "Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance" (PDF). OECD Series on Risk Management. Paris: OECD Publishing. p. 23. Archived from the original (PDF) on July 13, 2021.
- ^ Wang Z, Buser AM, Cousins IT, Demattio S, Drost W, Johansson O, et al. (December 2021). "A New OECD Definition for Per- and Polyfluoroalkyl Substances". Environmental Science & Technology. 55 (23): 15575–15578. Bibcode:2021EnST...5515575W. doi:10.1021/acs.est.1c06896. PMID 34751569. S2CID 243861839.
- ^ Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs) (Report). Series on Risk Management No. 39. OECD. Archived from the original on January 17, 2020. Retrieved December 9, 2019.
- ^ "PFAS structures in DSSTox (update August 2022)". CompTox Chemicals Dashboard. Washington, D.C.: U.S. Environmental Protection Agency (EPA). Retrieved October 21, 2022.
{{cite web}}: CS1 maint: url-status (link) "List consists of all DTXSID records with a structure assigned, and using a set of substructural filters based on community input." - ^ "PubChem Classification Browser – PFAS and Fluorinated Compounds in PubChem Tree". pubchem.ncbi.nlm.nih.gov. NBCI. Retrieved October 21, 2022.
- ^ a b Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC (June 2006). "Biological monitoring of polyfluoroalkyl substances: A review". Environmental Science & Technology. 40 (11): 3463–73. Bibcode:2006EnST...40.3463H. doi:10.1021/es052580b. PMID 16786681. Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC (2006). "Supporting Information" (PDF). Environmental Science & Technology. 40 (11): 3463–3473. Bibcode:2006EnST...40.3463H. doi:10.1021/es052580b. PMID 16786681. Archived from the original (PDF) on October 14, 2019.
- ^ "Per- and Polyfluorinated Substances (PFAS) Factsheet". National Biomonitoring Program. Centers for Disease Control and Prevention. September 2, 2021. Retrieved October 10, 2021.
- ^ Elton, Charlotte (February 24, 2023). "'Frightening' scale of Europe's forever chemical pollution revealed". euronews. Retrieved February 25, 2023.
- ^ "New Report Calls for Expanded PFAS Testing for People With History of Elevated Exposure, Offers Advice for Clinical Treatment". National Academies of Sciences, Engineering, and Medicine (NASEM). July 28, 2022. Retrieved August 4, 2022.
- ^ Perkins T (July 16, 2021). "Maine bans toxic 'forever chemicals' under groundbreaking new law". The Guardian. Archived from the original on July 16, 2021.
- ^ Lim XZ (August 27, 2021). "Maine's ban on 'forever chemicals' marks a big win for some scientists". Science. doi:10.1126/science.abm1382. Archived from the original on August 31, 2021. Retrieved August 31, 2021.
- ^ Salager JL (2002). "Surfactants-Types and Uses" (PDF). FIRP Booklet # 300-A. Universidad de los Andes Laboratory of Formulation, Interfaces Rheology, and Processes. p. 45. Archived (PDF) from the original on July 31, 2020. Retrieved September 7, 2008.
- ^ "Fluorosurfactant — Structure / Function". Mason Chemical Company. 2007. Archived from the original on July 5, 2008. Retrieved November 1, 2008.
- ^ a b c Renner R (January 2006). "The long and the short of perfluorinated replacements". Environmental Science & Technology. 40 (1): 12–3. Bibcode:2006EnST...40...12R. doi:10.1021/es062612a. PMID 16433328.
- ^ a b "Guide to PFAS in our environment debuts". C&EN Global Enterprise. 97 (21): 12. May 27, 2019. doi:10.1021/cen-09721-polcon2. ISSN 2474-7408. S2CID 199655540.
- ^ "Preliminary Lists of PFOS, PFAS, PFOA and Related Compounds and Chemicals that May Degrade to PFCA". OECD Papers. 6 (11): 1–194. October 25, 2006. doi:10.1787/oecd_papers-v6-art38-en. ISSN 1609-1914.
- ^ a b c d e Ubel FA, Sorenson SD, Roach DE (August 1980). "Health status of plant workers exposed to fluorochemicals--a preliminary report". American Industrial Hygiene Association Journal. 41 (8): 584–9. doi:10.1080/15298668091425310. PMID 7405826.
- ^ a b c d Olsen GW, Burris JM, Burlew MM, Mandel JH (March 2003). "Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations". Journal of Occupational and Environmental Medicine. 45 (3): 260–70. doi:10.1097/01.jom.0000052958.59271.10. PMID 12661183. S2CID 11648767.
- ^ "Emerging chemical risks in Europe — 'PFAS'". European Environment Agency. 2019. Archived from the original on February 6, 2020.
- ^ a b c d e f "Toxicological profile for Perfluoroalkyls". Agency for Toxic Substances and Disease Registry. 2018. Archived from the original on May 12, 2021.
- ^ "Some Chemicals Used as Solvents and in Polymer Manufacture". IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 110. 2016. Archived from the original on March 24, 2020.
- ^ Barry V, Winquist A, Steenland K (2013). "Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant". Environmental Health Perspectives. 121 (11–12): 1313–8. doi:10.1289/ehp.1306615. PMC 3855514. PMID 24007715.
- ^ Fenton SE, Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Hines EP, et al. (June 2009). "Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups". Reproductive Toxicology. 27 (3–4): 365–372. doi:10.1016/j.reprotox.2009.02.012. PMC 3446208. PMID 19429407.
- ^ White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE (August 2011). "Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice". Environmental Health Perspectives. 119 (8): 1070–6. doi:10.1289/ehp.1002741. PMC 3237341. PMID 21501981.
- ^ Swan SH, Colino S (February 2021). Count down: how our modern world is threatening sperm counts, altering male and female reproductive development, and imperiling the future of the human race. New York, USA: Scribner. ISBN 978-1-9821-1366-7.
- ^ Costello E, Rock S, Stratakis N, Eckel SP, Walker DI, Valvi D, et al. (April 2022). "Exposure to per- and Polyfluoroalkyl Substances and Markers of Liver Injury: A Systematic Review and Meta-Analysis". Environmental Health Perspectives. 130 (4): 46001. doi:10.1289/EHP10092. PMC 9044977. PMID 35475652.
- ^ a b c "C8 Science Panel". www.c8sciencepanel.org. Archived from the original on June 18, 2019. Retrieved June 8, 2019.
- ^ Steenland K, Jin C, MacNeil J, Lally C, Ducatman A, Vieira V, Fletcher T (July 2009). "Predictors of PFOA levels in a community surrounding a chemical plant". Environmental Health Perspectives. 117 (7): 1083–8. doi:10.1289/ehp.0800294. PMC 2717134. PMID 19654917.
- ^ "Probable Link Evaluation for heart disease (including high blood pressure, high cholesterol, coronary artery disease)" (PDF). C8 Science Panel. October 29, 2012.
- ^ "Probable Link Evaluation of Autoimmune Disease" (PDF). C8 Science Panel. July 30, 2012.
- ^ "Probable Link Evaluation of Thyroid disease" (PDF). C8 Science Panel. July 30, 2012.
- ^ "Probable Link Evaluation of Cancer" (PDF). C8 Science Panel. April 15, 2012.
- ^ "Probable Link Evaluation of Pregnancy Induced Hypertension and Preeclampsia" (PDF). C8 Science Panel. December 5, 2011.
- ^ Cousins IT, Johansson JH, Salter ME, Sha B, Scheringer M (August 2022). "Outside the Safe Operating Space of a New Planetary Boundary for Per- and Polyfluoroalkyl Substances (PFAS)". Environmental Science & Technology. 56 (16). American Chemical Society: 11172–11179. Bibcode:2022EnST...5611172C. doi:10.1021/acs.est.2c02765. PMC 9387091. PMID 35916421.
- ^ "Pollution: 'Forever chemicals' in rainwater exceed safe levels". BBC News. August 2, 2022. Retrieved September 14, 2022.
- ^ a b "Nordic Council of Ministers (2019). The cost of inaction. A socioeconomic analysis of environmental and health impacts linked to exposure" (PDF). Archived (PDF) from the original on October 1, 2019. Retrieved October 1, 2019.
- ^ Obsekov V, Kahn LG, Trasande L (July 26, 2022). "Leveraging Systematic Reviews to Explore Disease Burden and Costs of Per- and Polyfluoroalkyl Substance Exposures in the United States". Exposure and Health. doi:10.1007/s12403-022-00496-y. ISSN 2451-9766. S2CID 251072281.
- ^ a b Hosokawa M, Satoh T (October 1993). "Differences in the induction of carboxylesterase isozymes in rat liver microsomes by perfluorinated fatty acids". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 23 (10): 1125–33. doi:10.3109/00498259309059427. PMID 8259694.
- ^ DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, et al. (January 8, 2009). "Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha". Critical Reviews in Toxicology. 39 (1): 76–94. doi:10.1080/10408440802209804. PMID 18802816. S2CID 96896603.
- ^ DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR (November 22, 2011). "Immunotoxicity of perfluorinated compounds: recent developments". Toxicologic Pathology. 40 (2): 300–11. doi:10.1177/0192623311428473. PMID 22109712. S2CID 35549835.
- ^ Steenland K, Zhao L, Winquist A, Parks C (August 2013). "Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio valley". Environmental Health Perspectives. 121 (8): 900–5. doi:10.1289/ehp.1206449. PMC 3734500. PMID 23735465.
- ^ a b Lee JE, Choi K (March 2017). "Perfluoroalkyl substances exposure and thyroid hormones in humans: epidemiological observations and implications". Annals of Pediatric Endocrinology & Metabolism. 22 (1): 6–14. doi:10.6065/apem.2017.22.1.6. PMC 5401824. PMID 28443254.
- ^ a b Song M, Kim YJ, Park YK, Ryu JC (August 2012). "Changes in thyroid peroxidase activity in response to various chemicals". Journal of Environmental Monitoring. 14 (8): 2121–6. doi:10.1039/c2em30106g. PMID 22699773.
- ^ Barry V, Winquist A, Steenland K (2013). "Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant". Environmental Health Perspectives. 121 (11–12): 1313–8. doi:10.1289/ehp.1306615. PMC 3855514. PMID 24007715.
- ^ Klaunig JE, Hocevar BA, Kamendulis LM (July 2012). "Mode of Action analysis of perfluorooctanoic acid (PFOA) tumorigenicity and Human Relevance". Reproductive Toxicology. 33 (4): 410–418. doi:10.1016/j.reprotox.2011.10.014. PMID 22120428.
- ^ Li K, Gao P, Xiang P, Zhang X, Cui X, Ma LQ (February 2017). "Molecular mechanisms of PFOA-induced toxicity in animals and humans: Implications for health risks". Environment International. 99: 43–54. doi:10.1016/j.envint.2016.11.014. PMID 27871799.
- ^ Tonks DL (September 1994). "Percolation wave propagation, and void link-up effects in ductile fracture". Le Journal de Physique IV. 04 (C8): C8–665-C8-670. Bibcode:1994STIN...9511209T. doi:10.1051/jp4:19948101. ISSN 1155-4339. Archived from the original on June 25, 2021. Retrieved December 2, 2019.
- ^ a b Starling AP (2013). Perflourylalkyl substances in pregnancy and the risk of preeclampsia. University of North Carolina at Chapel Hill (Thesis). pp. 1–215. doi:10.17615/qhqh-4265.
- ^ a b "Agency for Toxic Substances and Disease Registry (ATSDR)", Health Care Policy and Politics a to Z, CQ Press, 2009, doi:10.4135/9781452240121.n18, ISBN 9780872897762
- ^ Hu Y, Liu G, Rood J, Liang L, Bray GA, de Jonge L, et al. (December 2019). "Perfluoroalkyl substances and changes in bone mineral density: A prospective analysis in the POUNDS-LOST study". Environmental Research. 179 (Pt A): 108775. Bibcode:2019ER....179j8775H. doi:10.1016/j.envres.2019.108775. PMC 6905427. PMID 31593837.
- ^ Donauer S, Chen A, Xu Y, Calafat AM, Sjodin A, Yolton K (March 2015). "Prenatal exposure to polybrominated diphenyl ethers and polyfluoroalkyl chemicals and infant neurobehavior". The Journal of Pediatrics. 166 (3): 736–42. doi:10.1016/j.jpeds.2014.11.021. PMC 4344877. PMID 25524317.
- ^ Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL (November 2007). "Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000". Environmental Health Perspectives. 115 (11): 1596–602. doi:10.1289/ehp.10598. PMC 2072821. PMID 18007991.
- ^ Wang Z, Cousins IT, Berger U, Hungerbühler K, Scheringer M (2016). "Comparative assessment of the environmental hazards of and exposure to perfluoroalkyl phosphonic and phosphinic acids (PFPAs and PFPiAs): Current knowledge, gaps, challenges and research needs". Environment International. 89–90: 235–47. doi:10.1016/j.envint.2016.01.023. PMID 26922149.[permanent dead link]
- ^ Blum A, Balan SA, Scheringer M, Trier X, Goldenman G, Cousins IT, et al. (May 2015). "The Madrid Statement on Poly- and Perfluoroalkyl Substances (PFASs)". Environmental Health Perspectives. 123 (5): A107-11. doi:10.1289/ehp.1509934. PMC 4421777. PMID 25932614.
- ^ "Stockholm Convention Clearing". chm.pops.int. Secretariat of the Stockholm Convention. Archived from the original on April 10, 2011. Retrieved October 26, 2016.
- ^ "Opinion | These toxic chemicals are everywhere — even in your body. And they won't ever go away". Washington Post. Archived from the original on May 9, 2019. Retrieved June 8, 2019.
- ^ Turkewitz J (February 22, 2019). "Toxic 'Forever Chemicals' in Drinking Water Leave Military Families Reeling". The New York Times. ISSN 0362-4331. Archived from the original on June 8, 2019. Retrieved June 8, 2019.
- ^ Kounang N (June 3, 2019). "FDA confirms PFAS chemicals are in the US food supply". CNN. Archived from the original on June 8, 2019. Retrieved June 8, 2019.
- ^ "Critics say EPA action plan on toxic 'forever chemicals' falls short". The Washington Post. February 14, 2019. Archived from the original on June 8, 2019. Retrieved June 8, 2019.
- ^ "Companies deny responsibility for toxic 'forever chemicals' contamination". The Guardian. 2019. Archived from the original on September 11, 2019.
- ^ Wang Z, Cousins IT, Scheringer M, Hungerbuehler K (February 2015). "Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions". Environment International. 75: 172–179. doi:10.1016/j.envint.2014.11.013. PMID 25461427.
- ^ Birnbaum LS, Grandjean P (May 2015). "Alternatives to PFASs: perspectives on the science". Environmental Health Perspectives. 123 (5): A104-5. doi:10.1289/ehp.1509944. PMC 4421778. PMID 25932670.
- ^ Perry MJ, Nguyen GN, Porter ND (2016). "The Current Epidemiologic Evidence on Exposures to Poly- and Perfluoroalkyl Substances (PFASs) and Male Reproductive Health". Current Epidemiology Reports. 3 (1): 19–26. doi:10.1007/s40471-016-0071-y. ISSN 2196-2995. S2CID 88276945.
- ^ Scheringer M, Trier X, Cousins IT, de Voogt P, Fletcher T, Wang Z, Webster TF (November 2014). "Helsingør statement on poly- and perfluorinated alkyl substances (PFASs)". Chemosphere. 114: 337–9. Bibcode:2014Chmsp.114..337S. doi:10.1016/j.chemosphere.2014.05.044. PMID 24938172.
- ^ "The "forever chemicals" that are harming our health: PFAS". Health and Environment Alliance. February 4, 2020. Archived from the original on February 6, 2020. Retrieved March 6, 2020.
- ^ Thomaidi VS, Tsahouridou A, Matsoukas C, Stasinakis AS, Petreas M, Kalantzi OI (April 2020). "Risk assessment of PFASs in drinking water using a probabilistic risk quotient methodology". The Science of the Total Environment. 712: 136485. Bibcode:2020ScTEn.712m6485T. doi:10.1016/j.scitotenv.2019.136485. PMID 31927447. S2CID 210167277.
- ^ Arvaniti OS, Stasinakis AS (August 2015). "Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment". The Science of the Total Environment. 524–525: 81–92. Bibcode:2015ScTEn.524...81A. doi:10.1016/j.scitotenv.2015.04.023. PMID 25889547.
- ^ Nika MC, Ntaiou K, Elytis K, Thomaidi VS, Gatidou G, Kalantzi OI, et al. (July 2020). "Wide-scope target analysis of emerging contaminants in landfill leachates and risk assessment using Risk Quotient methodology". Journal of Hazardous Materials. 394: 122493. doi:10.1016/j.jhazmat.2020.122493. PMID 32240898. S2CID 214766390.
- ^ Perkins T (December 18, 2021). "PFAS 'forever chemicals' constantly cycle through ground, air and water, study finds". The Guardian.
- ^ Sha B, Johansson JH, Tunved P, Bohlin-Nizzetto P, Cousins IT, Salter ME (January 2022). "Sea Spray Aerosol (SSA) as a Source of Perfluoroalkyl Acids (PFAAs) to the Atmosphere: Field Evidence from Long-Term Air Monitoring". Environmental Science & Technology. 56 (1). American Chemical Society (ACS): 228–238. Bibcode:2022EnST...56..228S. doi:10.1021/acs.est.1c04277. PMC 8733926. PMID 34907779.
- ^ a b Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP (November 2003). "Binding of perfluorinated fatty acids to serum proteins". Environmental Toxicology and Chemistry. 22 (11): 2639–2649. doi:10.1897/02-553. PMID 14587903. S2CID 15768654.
- ^ Martin JW, Mabury SA, Solomon KR, Muir DC (January 2003). "Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss)". Environmental Toxicology and Chemistry. 22 (1): 196–204. doi:10.1002/etc.5620220126. PMID 12503765. S2CID 12659454.
- ^ LaMotte, Sandee (January 17, 2023). "Locally caught fish are full of dangerous chemicals called PFAS, study finds". CNN. Archived from the original on February 14, 2023. Retrieved February 15, 2023.
- ^ Barbo, Nadia; Stoiber, Tasha; Naidenko, Olga V.; Andrews, David Q. (March 1, 2023). "Locally caught freshwater fish across the United States are likely a significant source of exposure to PFOS and other perfluorinated compounds". Environmental Research. 220: 115165. Bibcode:2023ER....220k5165B. doi:10.1016/j.envres.2022.115165. ISSN 0013-9351. PMID 36584847. S2CID 255248441.
- ^ Choi S, Kim JJ, Kim MH, Joo YS, Chung MS, Kho Y, Lee KW (June 2020). "Origin and organ-specific bioaccumulation pattern of perfluorinated alkyl substances in crabs". Environmental Pollution. 261: 114185. doi:10.1016/j.envpol.2020.114185. PMID 32114125. S2CID 211727091.
- ^ a b Fair PA, Wolf B, White ND, Arnott SA, Kannan K, Karthikraj R, Vena JE (April 2019). "Perfluoroalkyl substances (PFASs) in edible fish species from Charleston Harbor and tributaries, South Carolina, United States: Exposure and risk assessment". Environmental Research. 171: 266–277. Bibcode:2019ER....171..266F. doi:10.1016/j.envres.2019.01.021. PMC 6943835. PMID 30703622.
- ^ Teunen L, Bervoets L, Belpaire C, De Jonge M, Groffen T (March 29, 2021). "PFAS accumulation in indigenous and translocated aquatic organisms from Belgium, with translation to human and ecological health risk". Environmental Sciences Europe. 33 (1): 39. doi:10.1186/s12302-021-00477-z. ISSN 2190-4715. S2CID 232414650.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Taylor MD, Beyer-Robson J, Johnson DD, Knott NA, Bowles KC (June 2018). "Bioaccumulation of perfluoroalkyl substances in exploited fish and crustaceans: Spatial trends across two estuarine systems". Marine Pollution Bulletin. 131 (Pt A): 303–313. doi:10.1016/j.marpolbul.2018.04.029. PMID 29886951. S2CID 47009972.
- ^ Sun JM, Kelly BC, Gobas FA, Sunderland EM (August 2022). "A food web bioaccumulation model for the accumulation of per- and polyfluoroalkyl substances (PFAS) in fish: how important is renal elimination?". Environmental Science. Processes & Impacts. 24 (8): 1152–1164. doi:10.1039/D2EM00047D. PMC 9384792. PMID 35678632.
- ^ "'Shocked and disgusted' Katherine residents demand action on PFAS contamination". ABC News. October 10, 2017. Archived from the original on October 10, 2017. Retrieved October 10, 2017.
- ^ McLennan C (December 5, 2019). "Tindal's PFAS hot spots record startling results". Katherine Times. Archived from the original on February 21, 2020. Retrieved February 21, 2020.
- ^ Curtis K (April 21, 2022). "Airbases to get $428 million upgrade as government switches back to national security". The Sydney Morning Herald. Retrieved April 22, 2022.
- ^ O'Keeffe J. "Keeping Drinking Water Safe: New Guidelines for PFASs in Canada". National Collaborating Centre for Environmental Health. National Collaborating Centre for Environmental Health. Archived from the original on August 7, 2020. Retrieved July 22, 2020.
- ^ "Perfluoroalkylated substances in drinking water". canada.ca. Government of Canada. April 2019. Archived from the original on August 15, 2020. Retrieved July 22, 2020.
- ^ "Council Conclusions on Chemicals". European Council.
- ^ "PFAS". RIVM.
- ^ a b "ECHA publishes PFAS restriction proposal". ECHA. Retrieved February 8, 2023.
- ^ "Restriction procedure". ECHA. Retrieved February 8, 2023.
- ^ PFAS negli alimenti dell’area rossa del Veneto [https://www.greenpeace.org/static/planet4-italy-stateless/2021/09/7818ce34-pfas-negli-alimenti-dellarea-rossa-del-veneto.pdf
- ^ "Fact Sheet: 2010/2015 PFOA Stewardship Program". Assessing and Managing Chemicals under TSCA. EPA. August 9, 2018. Archived from the original on December 8, 2018.
- ^ "Basic Information on PFAS". EPA. December 6, 2018. Archived from the original on December 23, 2018.
- ^ Hahn SM (July 31, 2020). "FDA Announces Voluntary Agreement with Manufacturers to Phase Out Certain Short-Chain PFAS Used in Food Packaging". FDA. Archived from the original on August 2, 2020. Retrieved August 1, 2020.
- ^ a b "Toxic 'forever chemicals' widespread in top makeup brands, study finds". The Guardian. June 15, 2021. Archived from the original on July 7, 2021. Retrieved July 7, 2021.
- ^ Whitehead HD, Venier M, Wu Y, Eastman E, Urbanik S, Diamond ML, et al. (June 15, 2021). "Fluorinated Compounds in North American Cosmetics". Environmental Science & Technology Letters. 8 (7): 538–544. doi:10.1021/acs.estlett.1c00240. hdl:20.500.11850/495857. S2CID 236284279. Archived from the original on July 22, 2021. Retrieved July 11, 2021.
- ^ "Toxic 'Forever Chemicals' Widespread in Top Makeup Brands, Study Finds; Researchers Find Signs of PFAS in over Half of 231 Samples of Products Including Lipstick, Mascara and Foundation". The Guardian. UK. June 15, 2021. Archived from the original on June 26, 2021.
- ^ Root T (June 15, 2021). "Senate bill would ban toxic 'forever chemicals' in makeup, which new study found are often unlabeled". The Washington Post. Archived from the original on June 16, 2021. Retrieved July 2, 2021.
- ^ LaMotte S. "Makeup may contain potentially toxic chemicals called PFAS, study finds". CNN. Archived from the original on June 29, 2021. Retrieved July 7, 2021.
- ^ Perkins, Tom (January 13, 2023). "Bills to regulate toxic 'forever chemicals' died in Congress – with Republican help". The Guardian. ISSN 0261-3077. Retrieved February 15, 2023.
- ^ Timmis A (January 2018). "Using Dredged Materials to Improve a Salt Marsh". The Military Engineer. 110 (712): 61. Archived from the original on November 7, 2018. Retrieved December 18, 2018.
- ^ Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. (October 2016). "Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants". Environmental Science & Technology Letters. 3 (10): 344–350. doi:10.1021/acs.estlett.6b00260. PMC 5062567. PMID 27752509.
- ^ Scientific American, 22 Jan. 2021 "Forever Chemicals Are Widespread in U.S. Drinking Water: Experts Hope that with The Incoming Biden Administration, The Federal Government Will Finally Regulate A Class of Chemicals Known as PFASs"
- ^ "Drinking Water Health Advisories for PFOA and PFOS". EPA. December 9, 2020. Archived from the original on December 28, 2020. Retrieved December 27, 2020.
- ^ "Fact Sheet; PFOA & PFOS Drinking Water Health Advisories". November 2016. EPA 800-F-16-003. Archived from the original on December 26, 2020. Retrieved December 27, 2020.
- ^ EPA (2021-03-03). "Announcement of Final Regulatory Determinations for Contaminants on the Fourth Drinking Water Contaminant Candidate List." Federal Register, 86 FR 12272
- ^ EPA (2021-12-27). "Revisions to the Unregulated Contaminant Monitoring Rule (UCMR 5) for Public Water Systems and Announcement of Public Meetings." Federal Register, 86 FR 73131
- ^ "Fifth Unregulated Contaminant Monitoring Rule". EPA. February 22, 2022.
- ^ "Organic Chemicals, Plastics and Synthetic Fibers Effluent Guidelines". EPA. July 13, 2021.
- ^ "Metal Finishing Effluent Guidelines". EPA. September 24, 2021.
- ^ "PFAS Strategic Roadmap: EPA's Commitments to Action 2021-2024". EPA. January 27, 2022.
- ^ "Risk Assessment of Pollutants in Biosolids". EPA. May 3, 2022.
- ^ Maher K (June 15, 2022). "EPA Lowers Bar for Toxic Chemicals Contamination". The Wall Street Journal. Retrieved June 15, 2022.
- ^ washingtonpost.com/climate-environment/2022/08/26/forever-chemicals-epa-cleanup-rule/
- ^ "Meaningful and Achievable Steps You Can Take to Reduce Your Risk". PFOA, PFOS and Other PFAS. EPA. August 18, 2022.
- ^ Duggan T (October 5, 2021). "California bans PFAS chemicals from baby products and food packaging". San Francisco Chronicle.
- ^ "'I don't know how we'll survive': the farmers facing ruin in America's 'forever chemicals' crisis". The Guardian. Guardian News & Media Limited. March 22, 2022. Retrieved March 28, 2022.
- ^ 'Complete crisis' as PFAS discovery upends life and livelihood of young Maine farming family
- ^ a b c d e "FY 2020 Fast Facts". Michigan PFAS Action Response Team. Lansing, MI: Michigan Department of Environment, Great Lakes, and Energy. Archived from the original on December 18, 2018. Retrieved March 27, 2021.
- ^ "Dana Nessel sues 3M, DuPont over 'unconscionable' PFAS pollution in Michigan | Bridge Michigan". www.bridgemi.com. Retrieved March 28, 2022.
- ^ "EGLE - Michigan adopts strict PFAS in drinking water standards". www.michigan.gov. Archived from the original on March 10, 2022. Retrieved March 10, 2022.
- ^ Matheny K. "Michigan's drinking water standards for these chemicals now among toughest in nation". Detroit Free Press. Retrieved March 31, 2022.
- ^ "Toxic 'forever chemicals' found in Michigan farm's beef". abcNEWS. ABC News Internet Ventures. Retrieved March 28, 2022.
- ^ "3M Settles Minnesota Lawsuit for $850 Million". Bloomberg. June 7, 2019. Archived from the original on June 8, 2019. Retrieved June 8, 2019.
- ^ Fallon S (September 6, 2018). "New Jersey becomes first state to regulate dangerous chemical PFNA in drinking water". North Jersey Record. Woodland Park, NJ. Archived from the original on November 29, 2020. Retrieved December 27, 2020.
- ^ "Maximum Contaminant Levels (MCLs) for Perfluorononanoic Acid and 1,2,3-Trichloropropane; Private Well Testing for Arsenic, Gross Alpha Particle Activity, and Certain Synthetic Organic Compounds". Trenton, NJ: New Jersey Department of Environmental Protection (NJDEP). September 4, 2018. 50 N.J.R. 1939(a). Archived from the original on October 6, 2021. Retrieved December 27, 2020.
- ^ "Adoption of ground water quality standards and maximum contaminant levels for perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS)". NJDEP. June 1, 2020. Archived from the original on June 25, 2021. Retrieved December 27, 2020.
- ^ "AG Grewal, DEP Commissioner Announce 4 New Environmental Lawsuits Focused on Contamination Allegedly Linked to DuPont, Chemours, 3M". Totowa, NJ: New Jersey Office of the Attorney General. March 27, 2019. Press release. Archived from the original on January 13, 2021. Retrieved February 14, 2021.
- ^ Norton GP (April 17, 2019). "Re: Statewide PFAS Directive, Information Request and Notice to Insurers". Letter to Shawn LaTourette – via Internet Archive.
- ^ Warren MS (May 13, 2019). "State ordered chemical companies to pay for pollution clean-up. They say, no way!". NJ.com. NJ Advance Media. Archived from the original on October 19, 2019. Retrieved September 30, 2019.
- ^ Washington JW, Rosal CG, McCord JP, Strynar MJ, Lindstrom AB, Bergman EL, et al. (June 2020). "Nontargeted mass-spectral detection of chloroperfluoropolyether carboxylates in New Jersey soils". Science. 368 (6495): 1103–1107. Bibcode:2020Sci...368.1103W. doi:10.1126/science.aba7127. PMC 7814412. PMID 32499438.
- ^ McCord JP, Strynar MJ, Washington JW, Bergman EL, Goodrow SM (December 2020). "Emerging Chlorinated Polyfluorinated Polyether Compounds Impacting the Waters of Southwestern New Jersey Identified by Use of Nontargeted Analysis". Environmental Science & Technology Letters. 7 (12): 903–908. doi:10.1021/acs.estlett.0c00640. PMC 7863629. PMID 33553465.
- ^ New Jersey DEP plaintiffs v. Solvay Specialty Chemicals USA and Arkema Inc. defendants. GLO-L-001239-20. Trans ID 20202023975
- ^ "Solvay Launches Non-Fluorosurfactant Technologies in the U.S."
- ^ Joyce Dinglasan-Panlilio M, Prakash SS, Baker JE (January 2014). "Perfluorinated compounds in the surface waters of Puget Sound, Washington and Clayoquot and Barkley Sounds, British Columbia". Marine Pollution Bulletin. 78 (1–2): 173–80. doi:10.1016/j.marpolbul.2013.10.046. PMID 24262211.
- ^ Meador JP, Yeh A, Gallagher EP (November 2017). "Determining potential adverse effects in marine fish exposed to pharmaceuticals and personal care products with the fish plasma model and whole-body tissue concentrations". Environmental Pollution. 230: 1018–1029. doi:10.1016/j.envpol.2017.07.047. PMC 5595653. PMID 28764109.
- ^ Strivens JE, Kuo LJ, Liu Y, Noor KL (June 2021). "Spatial and temporal baseline of perfluorooctanesulfonic acid retained in sediment core samples from Puget Sound, Washington, USA". Marine Pollution Bulletin. 167: 112381. doi:10.1016/j.marpolbul.2021.112381. PMID 33962256. S2CID 233999063.
- ^ "DuPont settles lawsuits over leak of chemical used to make Teflon". Reuters. February 13, 2017. Archived from the original on June 8, 2019. Retrieved June 8, 2019.
- ^ "C8 Science Panel Website". www.c8sciencepanel.org. Archived from the original on April 14, 2013. Retrieved June 8, 2019.
- ^ Lerner S (October 6, 2018). "Nationwide class action lawsuit targets Dupont, Chemours, 3M, and other makers of PFAS chemicals". The Intercept. Archived from the original on October 7, 2018. Retrieved October 8, 2018.
- ^ Piña C (November 30, 2019). "'Dark Waters': 7 of the Film's Stars and Their Real-Life Inspirations". The Hollywood Reporter. Retrieved May 10, 2022.
- ^ Lerner S (July 31, 2018). "3M Knew about The Dangers of PFOA and PFOS Decades Ago, Internal Documents Show". The Intercept. First Look Media Works, Inc. Archived from the original on June 23, 2021. Retrieved May 2, 2020.
- ^ Halpern M (May 16, 2018). "Bipartisan Outrage as EPA, White House Try to Cover Up Chemical Health Assessment". Cambridge, MA: Union of Concerned Scientists. Archived from the original on March 5, 2020. Retrieved May 2, 2020.
- ^ Snider A (May 14, 2018). "White House, EPA headed off chemical pollution study". Politico. Archived from the original on May 16, 2018. Retrieved May 2, 2020.
- ^ "DoD: At Least 126 Bases Report Water Contaminants Linked to Cancer, Birth Defects". Military Times. April 26, 2018. Archived from the original on May 6, 2020.
- ^ Sullivan M (March 2018). "Addressing Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA)" (PDF).
- ^ The Guardian (UK), 23 Dec. 2022, "US Military ‘Downplayed’ The Number of Soldiers Exposed to ‘Forever Chemicals’--Analysis of Pentagon Report Reveals that Soldiers Exposed to PFAS Pollution at Much Higher Rate than Military Claims"
- ^ Glüge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D, et al. (October 2020). "An overview of the uses of per- and polyfluoroalkyl substances (PFAS)". Environmental Science. Processes & Impacts. 22 (12): 2345–2373. doi:10.1039/D0EM00291G. PMC 7784712. PMID 33125022.
- ^ Peaslee GF, Wilkinson JT, McGuinness SR, Tighe M, Caterisano N, Lee S, et al. (August 11, 2020). "Another Pathway for Firefighter Exposure to Per- and Polyfluoroalkyl Substances: Firefighter Textiles". Environmental Science & Technology Letters. 7 (8): 594–599. doi:10.1021/acs.estlett.0c00410. ISSN 2328-8930. S2CID 220481982. Archived from the original on October 20, 2020. Retrieved November 10, 2020.
- ^ a b c d Nilsson H, Kärrman A, Westberg H, Rotander A, van Bavel B, Lindström G (March 2010). "A time trend study of significantly elevated perfluorocarboxylate levels in humans after using fluorinated ski wax". Environmental Science & Technology. 44 (6): 2150–5. Bibcode:2010EnST...44.2150N. doi:10.1021/es9034733. PMID 20158198.
- ^ a b c Rotander A, Toms LM, Aylward L, Kay M, Mueller JF (September 2015). "Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF)". Environment International. 82: 28–34. doi:10.1016/j.envint.2015.05.005. PMID 26001497.
- ^ Trowbridge J, Gerona RR, Lin T, Rudel RA, Bessonneau V, Buren H, Morello-Frosch R (March 2020). "Exposure to Perfluoroalkyl Substances in a Cohort of Women Firefighters and Office Workers in San Francisco". Environmental Science & Technology. 54 (6): 3363–3374. Bibcode:2020EnST...54.3363T. doi:10.1021/acs.est.9b05490. PMC 7244264. PMID 32100527.
- ^ Tanner EM, Bloom MS, Wu Q, Kannan K, Yucel RM, Shrestha S, Fitzgerald EF (February 2018). "Occupational exposure to perfluoroalkyl substances and serum levels of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in an aging population from upstate New York: a retrospective cohort study". International Archives of Occupational and Environmental Health. 91 (2): 145–154. doi:10.1007/s00420-017-1267-2. PMID 29027000. S2CID 3950077. Archived from the original on October 6, 2021. Retrieved November 10, 2020.
- ^ Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D (May 2009). "Perfluorinated compounds--exposure assessment for the general population in Western countries". International Journal of Hygiene and Environmental Health. 212 (3): 239–70. doi:10.1016/j.ijheh.2008.04.007. PMID 18565792. Archived from the original on August 21, 2020. Retrieved November 10, 2020.
- ^ Kärrman A, Harada KH, Inoue K, Takasuga T, Ohi E, Koizumi A (May 2009). "Relationship between dietary exposure and serum perfluorochemical (PFC) levels--a case study". Environment International. 35 (4): 712–7. doi:10.1016/j.envint.2009.01.010. PMID 19250678. Archived from the original on June 15, 2018. Retrieved November 10, 2020.
- ^ a b c Costa G, Sartori S, Consonni D (March 2009). "Thirty years of medical surveillance in perfluooctanoic acid production workers". Journal of Occupational and Environmental Medicine. 51 (3): 364–72. doi:10.1097/JOM.0b013e3181965d80. PMID 19225424. S2CID 34813716. Archived from the original on October 6, 2021. Retrieved January 21, 2021.
- ^ Olsen GW, Burris JM, Burlew MM, Mandel JH (November 2000). "Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in ammonium perfluorooctanoate production workers". Drug and Chemical Toxicology. 23 (4): 603–20. doi:10.1081/DCT-100101973. PMID 11071397. S2CID 30289350. Archived from the original on July 5, 2021. Retrieved November 29, 2020.
- ^ a b Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR (September 2007). "Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers". Environmental Health Perspectives. 115 (9): 1298–305. doi:10.1289/ehp.10009. PMC 1964923. PMID 17805419.
- ^ Olsen GW, Burris JM, Mandel JH, Zobel LR (September 1999). "Serum perfluorooctane sulfonate and hepatic and lipid clinical chemistry tests in fluorochemical production employees". Journal of Occupational and Environmental Medicine. 41 (9): 799–806. doi:10.1097/00043764-199909000-00012. PMID 10491796. Archived from the original on October 6, 2021. Retrieved January 21, 2021.
- ^ a b Olsen GW, Chang SC, Noker PE, Gorman GS, Ehresman DJ, Lieder PH, Butenhoff JL (February 2009). "A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans". Toxicology. 256 (1–2): 65–74. doi:10.1016/j.tox.2008.11.008. PMID 19059455. Archived from the original on September 27, 2020. Retrieved November 29, 2020.
- ^ Olsen GW, Ehresman DJ, Buehrer BD, Gibson BA, Butenhoff JL, Zobel LR (August 2012). "Longitudinal assessment of lipid and hepatic clinical parameters in workers involved with the demolition of perfluoroalkyl manufacturing facilities". Journal of Occupational and Environmental Medicine. 54 (8): 974–83. doi:10.1097/JOM.0b013e31825461d2. PMID 22842914. S2CID 11478469. Archived from the original on October 6, 2021. Retrieved January 21, 2021.
- ^ Olsen GW, Zobel LR (November 2007). "Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers". International Archives of Occupational and Environmental Health. 81 (2): 231–46. doi:10.1007/s00420-007-0213-0. PMID 17605032. S2CID 25537444. Archived from the original on October 6, 2021. Retrieved November 29, 2020.
- ^ a b c d Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC (October 2007). "Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers". Journal of Occupational and Environmental Medicine. 49 (10): 1086–96. doi:10.1097/JOM.0b013e318156eca3. PMID 18000414. S2CID 20124680. Archived from the original on October 6, 2021. Retrieved January 21, 2021.
- ^ a b Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR (August 2007). "Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate". Journal of Occupational and Environmental Medicine. 49 (8): 872–9. doi:10.1097/JOM.0b013e318124a93f. PMID 17693785. S2CID 7339239. Archived from the original on October 6, 2021. Retrieved January 21, 2021.
- ^ Sakr CJ, Symons JM, Kreckmann KH, Leonard RC (October 2009). "Ischaemic heart disease mortality study among workers with occupational exposure to ammonium perfluorooctanoate". Occupational and Environmental Medicine. 66 (10): 699–703. doi:10.1136/oem.2008.041582. PMID 19553230. S2CID 30652104. Archived from the original on October 6, 2021. Retrieved November 29, 2020.
- ^ a b Steenland K, Zhao L, Winquist A (May 2015). "A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA)". Occupational and Environmental Medicine. 72 (5): 373–80. doi:10.1136/oemed-2014-102364. PMID 25601914. S2CID 28440634. Archived from the original on October 6, 2021. Retrieved November 29, 2020.
- ^ Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH (July 1998). "An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid". Journal of Occupational and Environmental Medicine. 40 (7): 614–22. doi:10.1097/00043764-199807000-00006. PMID 9675720. Archived from the original on October 6, 2021. Retrieved January 21, 2021.
- ^ Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, et al. (December 2003). "Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors". Environmental Health Perspectives. 111 (16): 1892–901. doi:10.1289/ehp.6316. PMC 1241763. PMID 14644663.
- ^ Olsen GW, Church TR, Larson EB, van Belle G, Lundberg JK, Hansen KJ, et al. (March 2004). "Serum concentrations of perfluorooctanesulfonate and other fluorochemicals in an elderly population from Seattle, Washington". Chemosphere. 54 (11): 1599–611. Bibcode:2004Chmsp..54.1599O. doi:10.1016/j.chemosphere.2003.09.025. PMID 14675839. Archived from the original on June 14, 2018. Retrieved November 29, 2020.
- ^ Olsen GW, Church TR, Hansen KJ, Burris JM, Butenhoff JL, Mandel JH, Zobel LR (January 1, 2004). "Quantitative Evaluation of Perfluorooctanesulfonate (PFOS) and Other Fluorochemicals in the Serum of Children". Journal of Children's Health. 2 (1): 53–76. doi:10.3109/15417060490447378. ISSN 1541-7069.
- ^ Olsen GW, Huang HY, Helzlsouer KJ, Hansen KJ, Butenhoff JL, Mandel JH (May 2005). "Historical comparison of perfluorooctanesulfonate, perfluorooctanoate, and other fluorochemicals in human blood". Environmental Health Perspectives. 113 (5): 539–45. doi:10.1289/ehp.7544. PMC 1257544. PMID 15866760.
- ^ Kubwabo C, Vais N, Benoit FM (June 2004). "A pilot study on the determination of perfluorooctanesulfonate and other perfluorinated compounds in blood of Canadians". Journal of Environmental Monitoring. 6 (6): 540–5. doi:10.1039/b314085g. PMID 15173906. Archived from the original on October 6, 2021. Retrieved November 29, 2020.
- ^ Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, et al. (September 2004). "Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries". Environmental Science & Technology. 38 (17): 4489–95. Bibcode:2004EnST...38.4489K. doi:10.1021/es0493446. PMID 15461154. Archived from the original on February 4, 2021. Retrieved November 29, 2020.
- ^ Harada K, Saito N, Inoue K, Yoshinaga T, Watanabe T, Sasaki S, et al. (March 2004). "The influence of time, sex and geographic factors on levels of perfluorooctane sulfonate and perfluorooctanoate in human serum over the last 25 years". Journal of Occupational Health. 46 (2): 141–7. doi:10.1539/joh.46.141. PMID 15090689. S2CID 9418835. Archived from the original on June 25, 2021. Retrieved November 29, 2020.
- ^ Fu J, Gao Y, Cui L, Wang T, Liang Y, Qu G, et al. (December 2016). "Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (PFAAs) in occupational workers in China". Scientific Reports. 6 (1): 38039. Bibcode:2016NatSR...638039F. doi:10.1038/srep38039. PMC 5131319. PMID 27905562.
- ^ Fu J, Gao Y, Wang T, Liang Y, Zhang A, Wang Y, Jiang G (March 2015). "Elevated levels of perfluoroalkyl acids in family members of occupationally exposed workers: the importance of dust transfer". Scientific Reports. 5 (1): 9313. Bibcode:2015NatSR...5E9313F. doi:10.1038/srep09313. PMC 5380130. PMID 25791573.
- ^ Gao Y, Fu J, Cao H, Wang Y, Zhang A, Liang Y, et al. (June 2015). "Differential accumulation and elimination behavior of perfluoroalkyl Acid isomers in occupational workers in a manufactory in China". Environmental Science & Technology. 49 (11): 6953–62. Bibcode:2015EnST...49.6953G. doi:10.1021/acs.est.5b00778. PMID 25927957. S2CID 23947500. Archived from the original on October 6, 2021. Retrieved December 2, 2019.
- ^ Lu Y, Gao K, Li X, Tang Z, Xiang L, Zhao H, et al. (August 2019). "Mass Spectrometry-Based Metabolomics Reveals Occupational Exposure to Per- and Polyfluoroalkyl Substances Relates to Oxidative Stress, Fatty Acid β-Oxidation Disorder, and Kidney Injury in a Manufactory in China". Environmental Science & Technology. 53 (16): 9800–9809. Bibcode:2019EnST...53.9800L. doi:10.1021/acs.est.9b01608. PMID 31246438. S2CID 195762433.
- ^ Wang Y, Fu J, Wang T, Liang Y, Pan Y, Cai Y, Jiang G (November 2010). "Distribution of perfluorooctane sulfonate and other perfluorochemicals in the ambient environment around a manufacturing facility in China". Environmental Science & Technology. 44 (21): 8062–7. Bibcode:2010EnST...44.8062W. doi:10.1021/es101810h. PMID 20879709. Archived from the original on June 25, 2021. Retrieved January 21, 2021.
- ^ a b Laitinen JA, Koponen J, Koikkalainen J, Kiviranta H (December 2014). "Firefighters' exposure to perfluoroalkyl acids and 2-butoxyethanol present in firefighting foams". Toxicology Letters. 231 (2): 227–32. doi:10.1016/j.toxlet.2014.09.007. PMID 25447453.
- ^ Jin C, Sun Y, Islam A, Qian Y, Ducatman A (March 2011). "Perfluoroalkyl acids including perfluorooctane sulfonate and perfluorohexane sulfonate in firefighters". Journal of Occupational and Environmental Medicine. 53 (3): 324–8. doi:10.1097/jom.0b013e31820d1314. PMID 21346631. S2CID 41993931.
- ^ Barton KE, Starling AP, Higgins CP, McDonough CA, Calafat AM, Adgate JL (January 2020). "Sociodemographic and behavioral determinants of serum concentrations of per- and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water". International Journal of Hygiene and Environmental Health. 223 (1): 256–266. doi:10.1016/j.ijheh.2019.07.012. PMC 6878185. PMID 31444118.
- ^ Colorado economic impact study on the Uranium Mill Tailings Remedial Action Project in Colorado: Colorado state fiscal year 1993 (Report). November 12, 1993. doi:10.2172/10112187. Archived from the original on June 25, 2021. Retrieved December 2, 2019.
- ^ "Toxics in firefighting". ecology.wa.gov. Retrieved January 14, 2022.
{{cite web}}: CS1 maint: url-status (link) - ^ a b c Tao L, Kannan K, Aldous KM, Mauer MP, Eadon GA (May 2008). "Biomonitoring of perfluorochemicals in plasma of New York State personnel responding to the World Trade Center disaster". Environmental Science & Technology. 42 (9): 3472–8. Bibcode:2008EnST...42.3472T. doi:10.1021/es8000079. PMID 18522136. Archived from the original on October 6, 2021. Retrieved December 2, 2019.
- ^ "Treatment Technologies". PFAS — Per- and Polyfluoroalkyl Substances. Washington, DC: Interstate Technology & Regulatory Council (ITRC). September 2020. Archived from the original on March 27, 2021. Retrieved March 27, 2021.
- ^ a b Fischer L (January 31, 2022). "How to Destroy 'Forever Chemicals'". Scientific American.
- ^ "PFAS Assessment & Mitigation". Columbus, OH: Battelle Memorial Institute. Archived from the original on December 18, 2018. Retrieved December 18, 2018.
- ^ a b Cameron L (October 9, 2018). "Diamond technology cleans up PFAS-contaminated wastewater". MSU Today. Michigan State University. Archived from the original on December 19, 2018. Retrieved December 18, 2018.
- ^ Mandelbaum RF (September 18, 2019). "A New Jersey Soil Bacteria Is First to Break Down Toxic 'Forever Chemical'". Gizmodo. Archived from the original on September 20, 2019. Retrieved September 19, 2019.
- ^ Lim XZ. "Can microbes save us from PFAS?". cen.acs.org.
- ^ Trang B, Li Y, Xue XS, Ateia M, Houk KN, Dichtel WR (August 2022). "Low-temperature mineralization of perfluorocarboxylic acids". Science. 377 (6608): 839–845. Bibcode:2022Sci...377..839T. doi:10.1126/science.abm8868. PMID 35981038.
- ^ Abbas M, Maceda AM, Firouzi HR, Xiao Z, Arman HD, Shi Y, et al. (December 2022). "Fluorine extraction from organofluorine molecules to make fluorinated clusters in yttrium MOFs". Chemical Science. 13 (48): 14285–14291. doi:10.1039/D2SC05143E. PMC 9749115. PMID 36545134.
- ^ Wen Y, Rentería-Gómez Á, Day GS, Smith MF, Yan TH, Ozdemir RO, et al. (July 2022). "Integrated Photocatalytic Reduction and Oxidation of Perfluorooctanoic Acid by Metal-Organic Frameworks: Key Insights into the Degradation Mechanisms". Journal of the American Chemical Society. 144 (26): 11840–11850. doi:10.1021/jacs.2c04341. PMID 35732040. S2CID 249956841.
- ^ "Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS): Frequently Asked Questions" (PDF). Centers for Disease Control. August 22, 2017. Archived (PDF) from the original on October 18, 2020. Retrieved August 13, 2019.
- ^ "ORD subset of PFAS with ongoing work methods; CompTox Chemicals Dashboard" (PDF). EPA. March 11, 2019. Archived (PDF) from the original on July 15, 2019. Retrieved August 13, 2019.
Further reading
- Lindstrom AB, Strynar MJ, Libelo EL (October 2011). "Polyfluorinated compounds: past, present, and future". Environmental Science & Technology. 45 (19): 7954–61. Bibcode:2011EnST...45.7954L. doi:10.1021/es2011622. PMID 21866930. S2CID 206946893. Archived from the original on October 6, 2021. Retrieved December 2, 2019.
- Ritter SK (2015). "The Shrinking Case For Fluorochemicals". Chemical & Engineering News. 93 (28): 27–29. doi:10.1021/cen-09328-scitech1. Archived from the original on August 18, 2016. Retrieved August 3, 2016.
- Lehmler HJ (March 2005). "Synthesis of environmentally relevant fluorinated surfactants--a review". Chemosphere. 58 (11): 1471–96. Bibcode:2005Chmsp..58.1471L. doi:10.1016/j.chemosphere.2004.11.078. PMID 15694468.
External links
- Per- and Polyfluoroalkyl Substances (PFAS) at the National Toxicology Program
- Per- and Polyfluoroalkyl Substances and Your Health at the Agency for Toxic Substances and Disease Registry
- Per- and Polyfluoroalkyl Substances (PFAS) at the United States Environmental Protection Agency
- Perfluoroalkyl chemicals (PFAS) at the European Chemicals Agency
- PFAS Contamination [map] in the U.S. by the Environmental Working Group
- Per- and Polyfluoroalkyl substances, National Institute for Occupational Safety and Health
- The Forever Pollution Project - Journalists tracking PFAS across Europe
- PFAS contamination in Queensland, Australia, State Library of Queensland
