Henipavirus: Difference between revisions
Added reference that experimentally demonstrates Nipah conforming to "rule of six" using a minigenome replication assay, this is direct evidence in support of the statement made in this sentence. |
Updated information of the roles of P protein products V, W, and C. Corrected the statement that "V, W, and C" are produced by RNA editing. This is incorrect. Only V and W are produced by RNA editing, C is produced through leaky scanning of the host ribosome, and is translated as a result of an alternate open reading frame. Appropriate citations have been added. |
||
| Line 36: | Line 36: | ||
In common with other members of the ''[[Paramyxoviridae]]'' family, the number of [[nucleotide]]s in the henipavirus genome is a multiple of six, consistent with what is known as the '[[Rule of six (viruses)|rule of six]]'.<ref>{{Cite journal |last=Halpin |first=Kim |last2=Bankamp |first2=Bettina |last3=Harcourt |first3=Brian H. |last4=Bellini |first4=William J. |last5=Rota |first5=Paul A.YR 2004 |title=Nipah virus conforms to the rule of six in a minigenome replication assay |url=https://www.microbiologyresearch.org/content/journal/jgv/10.1099/vir.0.19685-0 |journal=Journal of General Virology |volume=85 |issue=3 |pages=701–707 |doi=10.1099/vir.0.19685-0 |issn=1465-2099}}</ref><ref>{{cite journal |last1=Kolakofsky |first1=D |author2=Pelet, T |author3=Garcin, D |author4=Hausmann, S |author5=Curran, J |author6=Roux, L |title=Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited|journal=Journal of Virology|date=February 1998|volume=72|issue=2|pages=891–9|pmid=9444980|pmc=124558|doi=10.1128/JVI.72.2.891-899.1998 }}</ref> Deviation from the rule of six, through mutation or incomplete genome synthesis, leads to inefficient viral replication, probably due to structural constraints imposed by the binding between the RNA and the N protein. |
In common with other members of the ''[[Paramyxoviridae]]'' family, the number of [[nucleotide]]s in the henipavirus genome is a multiple of six, consistent with what is known as the '[[Rule of six (viruses)|rule of six]]'.<ref>{{Cite journal |last=Halpin |first=Kim |last2=Bankamp |first2=Bettina |last3=Harcourt |first3=Brian H. |last4=Bellini |first4=William J. |last5=Rota |first5=Paul A.YR 2004 |title=Nipah virus conforms to the rule of six in a minigenome replication assay |url=https://www.microbiologyresearch.org/content/journal/jgv/10.1099/vir.0.19685-0 |journal=Journal of General Virology |volume=85 |issue=3 |pages=701–707 |doi=10.1099/vir.0.19685-0 |issn=1465-2099}}</ref><ref>{{cite journal |last1=Kolakofsky |first1=D |author2=Pelet, T |author3=Garcin, D |author4=Hausmann, S |author5=Curran, J |author6=Roux, L |title=Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited|journal=Journal of Virology|date=February 1998|volume=72|issue=2|pages=891–9|pmid=9444980|pmc=124558|doi=10.1128/JVI.72.2.891-899.1998 }}</ref> Deviation from the rule of six, through mutation or incomplete genome synthesis, leads to inefficient viral replication, probably due to structural constraints imposed by the binding between the RNA and the N protein. |
||
Three additional protein products are produced from the henipavirus P gene: V, W, and C. The V and W proteins are generated through an unusual process called [[RNA editing]]. This specific process in henipaviruses involves the insertion of extra [[guanosine]] residues into the P gene [[mRNA]] prior to [[translation (genetics)|translation]]. The addition of a single guanosine results in production of V, and the addition of two guanosines residues produces W<ref>{{Cite journal |last=Shaw |first=Megan L. |date=2009-12 |title=Henipaviruses Employ a Multifaceted Approach to Evade the Antiviral Interferon Response |url=https://www.mdpi.com/1999-4915/1/3/1190 |journal=Viruses |language=en |volume=1 |issue=3 |pages=1190–1203 |doi=10.3390/v1031190 |issn=1999-4915 |pmc=PMC3185527 |pmid=21994589}}</ref>. The C protein is not produced through RNA editing but instead by [[leaky scanning]] of the host cell ribosome during translation of viral mRNA. P, V, and W possess an alternate open reading frame which results in production of C. P, V, W, and C are known to disrupt the host innate antiviral immune response through several different mechanisms<ref>{{Cite journal |last=Lawrence |first=Philip |last2=Escudero-Pérez |first2=Beatriz |date=2022-04-29 |title=Henipavirus Immune Evasion and Pathogenesis Mechanisms: Lessons Learnt from Natural Infection and Animal Models |url=https://pubmed.ncbi.nlm.nih.gov/35632678 |journal=Viruses |volume=14 |issue=5 |pages=936 |doi=10.3390/v14050936 |issn=1999-4915 |pmc=9146692 |pmid=35632678}}</ref>. P, V, and W contain [[STAT1]] binding domains, and act as [[interferon]] antagonists by sequestering STAT1 in the nucleus and cytoplasm<ref>{{Cite journal |last=Shaw |first=Megan L. |last2=García-Sastre |first2=Adolfo |last3=Palese |first3=Peter |last4=Basler |first4=Christopher F. |date=2004-06 |title=Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively |url=https://pubmed.ncbi.nlm.nih.gov/15140960 |journal=Journal of Virology |volume=78 |issue=11 |pages=5633–5641 |doi=10.1128/JVI.78.11.5633-5641.2004 |issn=0022-538X |pmc=PMC415790 |pmid=15140960}}</ref>. The C protein controls the early pro-inflammatory response<ref>{{Cite journal |last=Lo |first=Michael K. |last2=Peeples |first2=Mark E. |last3=Bellini |first3=William J. |last4=Nichol |first4=Stuart T. |last5=Rota |first5=Paul A. |last6=Spiropoulou |first6=Christina F. |date=2012-10-19 |title=Distinct and Overlapping Roles of Nipah Virus P Gene Products in Modulating the Human Endothelial Cell Antiviral Response |url=https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0047790 |journal=PLOS ONE |language=en |volume=7 |issue=10 |pages=e47790 |doi=10.1371/journal.pone.0047790 |issn=1932-6203 |pmc=PMC3477106 |pmid=23094089}}</ref> and is also known to promote the viral budding process via a [[ESCRT]]-dependent pathway<ref>{{Cite journal |last=Park |first=Arnold |last2=Yun |first2=Tatyana |last3=Vigant |first3=Frederic |last4=Pernet |first4=Olivier |last5=Won |first5=Sohui T. |last6=Dawes |first6=Brian E. |last7=Bartkowski |first7=Wojciech |last8=Freiberg |first8=Alexander N. |last9=Lee |first9=Benhur |date=2016-05-20 |title=Nipah Virus C Protein Recruits Tsg101 to Promote the Efficient Release of Virus in an ESCRT-Dependent Pathway |url=https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1005659 |journal=PLOS Pathogens |language=en |volume=12 |issue=5 |pages=e1005659 |doi=10.1371/journal.ppat.1005659 |issn=1553-7374}}</ref>. |
|||
Henipaviruses employ an unusual process called [[RNA editing]] to generate multiple proteins from a single gene. The specific process in henipaviruses involves the insertion of extra [[guanosine]] residues into the P gene [[mRNA]] prior to [[translation (genetics)|translation]]. The number of residues added determines whether the P, V C, or W proteins are synthesised. The functions of the V and W proteins are unknown, but they may be involved in disrupting host antiviral mechanisms. |
|||
== Life Cycle == |
== Life Cycle == |
||
Cell receptor ephrin-B2, which is located on epithelial cells around smaller arteries, neurons, and smooth muscle cells, |
Cell receptor ephrin-B2, which is located on epithelial cells around smaller arteries, neurons, and smooth muscle cells, is targeted by the viral protein G.<ref>{{Cite journal |last1=Bonaparte |first1=Matthew I. |last2=Dimitrov |first2=Antony S. |last3=Bossart |first3=Katharine N. |last4=Crameri |first4=Gary |last5=Mungall |first5=Bruce A. |last6=Bishop |first6=Kimberly A. |last7=Choudhry |first7=Vidita |last8=Dimitrov |first8=Dimiter S. |last9=Wang |first9=Lin-Fa |last10=Eaton |first10=Bryan T. |last11=Broder |first11=Christopher C. |date=2005-07-05 |title=Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus |journal=Proceedings of the National Academy of Sciences |volume=102 |issue=30 |pages=10652–10657 |doi=10.1073/pnas.0504887102 |pmid=15998730 |pmc=1169237 |bibcode=2005PNAS..10210652B |issn=0027-8424|doi-access=free }}</ref> Once the protein G binds to ephrin-B2, the viral protein F facilitates fusion with the host cell membrane and releases viral RNA into the host cell cytoplasm.<ref>{{Cite journal |last=Zuckerman |first=Arie J. |date=1996-06-10 |title=Fields virology, 3rd edn. (two vol. set): Edited by B.N. Fields, D.M. Knipe, P.M. Howley, R.M. Chanock, J.L. Melnick, T.P. Monath, B. Roizman and S.E. Straus, Lippincott-Raven, Philadelphia, P |url=http://doi.wiley.com/10.1016/0014-5793%2896%2988179-8 |journal=FEBS Letters |language=en |volume=388 |issue=1 |pages=88 |doi=10.1016/0014-5793(96)88179-8}}</ref> Upon entry, transcription of viral mRNA takes place using the viral RNA as a template. This process is started and stopped by the polymerase complex. Viral proteins are gathering in the cell as transcription occurs until the polymerase complex stops transcription and starts genome replication. Transcription of the viral RNA makes positive sense strands of RNA, which are then used as templates to make more negative sense viral RNA . Genome replication is halted before the viral particles can assemble to make a virion. Once the cell membrane is ready, new virions exit the host cell through budding.<ref>{{Citation |last1=Rota |first1=Paul A. |title=Molecular Virology of the Henipaviruses |date=2012 |url=http://link.springer.com/10.1007/82_2012_211 |work=Henipavirus |volume=359 |pages=41–58 |editor-last=Lee |editor-first=Benhur |place=Berlin, Heidelberg |publisher=Springer Berlin Heidelberg |doi=10.1007/82_2012_211 |isbn=978-3-642-29818-9 |access-date=2022-12-04 |last2=Lo |first2=Michael K. |pmid=22552699 |editor2-last=Rota |editor2-first=Paul A.}}</ref> |
||
== Vaccine == |
== Vaccine == |
||
Henipaviruses have high mortality rates in mammalian hosts, both human and animal. Because of this, there is a need for immunization against HeV and NiV. Both diseases can be transmitted from animals to humans and can cause respiratory and neurological issues.<ref>{{Cite journal |last1=Wong |first1=K. Thong |last2=Grosjean |first2=Isabelle |last3=Brisson |first3=Christine |last4=Blanquier |first4=Barissa |last5=Fevre-Montange |first5=Michelle |last6=Bernard |first6=Arlette |last7=Loth |first7=Philippe |last8=Georges-Courbot |first8=Marie-Claude |last9=Chevallier |first9=Michelle |last10=Akaoka |first10=Hideo |last11=Marianneau |first11=Philippe |last12=Lam |first12=Sai Kit |last13=Wild |first13=T. Fabian |last14=Deubel |first14=Vincent |date=November 2003 |title=A Golden Hamster Model for Human Acute Nipah Virus Infection |url=http://dx.doi.org/10.1016/s0002-9440(10)63569-9 |journal=The American Journal of Pathology |volume=163 |issue=5 |pages=2127–2137 |doi=10.1016/s0002-9440(10)63569-9 |pmid=14578210 |pmc=1892425 |issn=0002-9440}}</ref> HeV and NiV appear as encephalitis or severe respiratory illnesses in human hosts. Animal hosts also experience respiratory distress and occasionally fever and neurological disease. Other vaccines for viruses in the Paramyxoviridae family use neutralizing antibodies directed to bind to the surface glycoproteins. A vaccine for henipaviruses has still not been made, but would probably follow the same model as other vaccines for Paramyxoviridae family viruses.<ref>{{Citation |last1=Broder |first1=Christopher C. |title=Immunization Strategies Against Henipaviruses |date=2012 |work=Henipavirus |volume=359 |pages=197–223 |editor-last=Lee |editor-first=Benhur |place=Berlin, Heidelberg |publisher=Springer Berlin Heidelberg |doi=10.1007/82_2012_213 |isbn=978-3-642-29818-9 |pmc=4465348 |pmid=22481140 |last2=Geisbert |first2=Thomas W. |last3=Xu |first3=Kai |last4=Nikolov |first4=Dimitar B. |last5=Wang |first5=Lin-Fa |last6=Middleton |first6=Deborah |last7=Pallister |first7=Jackie |last8=Bossart |first8=Katharine N. |editor2-last=Rota |editor2-first=Paul A.}}</ref> |
Henipaviruses have high mortality rates in mammalian hosts, both human and animal. Because of this, there is a need for immunization against HeV and NiV. Both diseases can be transmitted from animals to humans and can cause respiratory and neurological issues.<ref>{{Cite journal |last1=Wong |first1=K. Thong |last2=Grosjean |first2=Isabelle |last3=Brisson |first3=Christine |last4=Blanquier |first4=Barissa |last5=Fevre-Montange |first5=Michelle |last6=Bernard |first6=Arlette |last7=Loth |first7=Philippe |last8=Georges-Courbot |first8=Marie-Claude |last9=Chevallier |first9=Michelle |last10=Akaoka |first10=Hideo |last11=Marianneau |first11=Philippe |last12=Lam |first12=Sai Kit |last13=Wild |first13=T. Fabian |last14=Deubel |first14=Vincent |date=November 2003 |title=A Golden Hamster Model for Human Acute Nipah Virus Infection |url=http://dx.doi.org/10.1016/s0002-9440(10)63569-9 |journal=The American Journal of Pathology |volume=163 |issue=5 |pages=2127–2137 |doi=10.1016/s0002-9440(10)63569-9 |pmid=14578210 |pmc=1892425 |issn=0002-9440}}</ref> HeV and NiV appear as encephalitis or severe respiratory illnesses in human hosts. Animal hosts also experience respiratory distress and occasionally fever and neurological disease. Other vaccines for viruses in the Paramyxoviridae family use neutralizing antibodies directed to bind to the surface glycoproteins. A human vaccine for henipaviruses has still not been made, but would probably follow the same model as other vaccines for Paramyxoviridae family viruses.<ref>{{Citation |last1=Broder |first1=Christopher C. |title=Immunization Strategies Against Henipaviruses |date=2012 |work=Henipavirus |volume=359 |pages=197–223 |editor-last=Lee |editor-first=Benhur |place=Berlin, Heidelberg |publisher=Springer Berlin Heidelberg |doi=10.1007/82_2012_213 |isbn=978-3-642-29818-9 |pmc=4465348 |pmid=22481140 |last2=Geisbert |first2=Thomas W. |last3=Xu |first3=Kai |last4=Nikolov |first4=Dimitar B. |last5=Wang |first5=Lin-Fa |last6=Middleton |first6=Deborah |last7=Pallister |first7=Jackie |last8=Bossart |first8=Katharine N. |editor2-last=Rota |editor2-first=Paul A.}}</ref> |
||
==Causes of emergence== |
==Causes of emergence== |
||
Revision as of 06:38, 29 March 2023
| Henipavirus | |
|---|---|
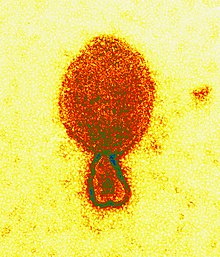
| |
| Colored transmission electron micrograph of a Hendra henipavirus virion (ca. 300 nm length) | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Negarnaviricota |
| Class: | Monjiviricetes |
| Order: | Mononegavirales |
| Family: | Paramyxoviridae |
| Subfamily: | Orthoparamyxovirinae |
| Genus: | Henipavirus |
| Species | |
Henipavirus is a genus of negative-strand RNA viruses in the family Paramyxoviridae, order Mononegavirales containing six established species,[1][2] and numerous others still under study.[3] Henipaviruses are naturally harboured by several species of small mammals, notably pteropid fruit bats (flying foxes), microbats of several species,[4] and shrews.[5][6] Henipaviruses are characterised by long genomes and a wide host range. Their recent emergence as zoonotic pathogens capable of causing illness and death in domestic animals and humans is a cause of concern.[7][8]
In 2009, RNA sequences of three novel viruses in phylogenetic relationship to known henipaviruses were detected in African straw-colored fruit bats (Eidolon helvum) in Ghana. The finding of these novel henipaviruses outside Australia and Asia indicates that the region of potential endemicity of henipaviruses may be worldwide.[9] These African henipaviruses are slowly being characterised.[10]
Nipah and Hendra henipaviruses are both considered category C (USDA-HHS overlap) select agents.[11]
Structure

Henipavirions are pleomorphic (variably shaped), ranging in size from 40 to 600 nm in diameter.[12] They possess a lipid membrane overlying a shell of viral matrix protein. At the core is a single helical strand of genomic RNA tightly bound to N (nucleocapsid) protein and associated with the L (large) and P (phosphoprotein) proteins, which provide RNA polymerase activity during replication.
Embedded within the lipid membrane are spikes of F (fusion) protein trimers and G (attachment) protein tetramers. The function of the G protein (except in the case of MojV-G) is to attach the virus to the surface of a host cell via Ephrin B1, B2, or B3, a family of highly conserved mammalian proteins.[13][14][15] The structure of the attachment glycoprotein has been determined by X-ray crystallography.[16] The F protein fuses the viral membrane with the host cell membrane, releasing the virion contents into the cell. It also causes infected cells to fuse with neighbouring cells to form large, multinucleated syncytia.
Genome
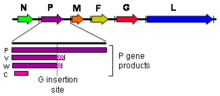
As all mononegaviral genomes, Hendra virus and Nipah virus genomes are non-segmented, single-stranded negative-sense RNA. Both genomes are 18.2 kb in length and contain six genes corresponding to six structural proteins.[17]
In common with other members of the Paramyxoviridae family, the number of nucleotides in the henipavirus genome is a multiple of six, consistent with what is known as the 'rule of six'.[18][19] Deviation from the rule of six, through mutation or incomplete genome synthesis, leads to inefficient viral replication, probably due to structural constraints imposed by the binding between the RNA and the N protein.
Three additional protein products are produced from the henipavirus P gene: V, W, and C. The V and W proteins are generated through an unusual process called RNA editing. This specific process in henipaviruses involves the insertion of extra guanosine residues into the P gene mRNA prior to translation. The addition of a single guanosine results in production of V, and the addition of two guanosines residues produces W[20]. The C protein is not produced through RNA editing but instead by leaky scanning of the host cell ribosome during translation of viral mRNA. P, V, and W possess an alternate open reading frame which results in production of C. P, V, W, and C are known to disrupt the host innate antiviral immune response through several different mechanisms[21]. P, V, and W contain STAT1 binding domains, and act as interferon antagonists by sequestering STAT1 in the nucleus and cytoplasm[22]. The C protein controls the early pro-inflammatory response[23] and is also known to promote the viral budding process via a ESCRT-dependent pathway[24].
Life Cycle
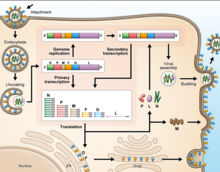
Cell receptor ephrin-B2, which is located on epithelial cells around smaller arteries, neurons, and smooth muscle cells, is targeted by the viral protein G.[25] Once the protein G binds to ephrin-B2, the viral protein F facilitates fusion with the host cell membrane and releases viral RNA into the host cell cytoplasm.[26] Upon entry, transcription of viral mRNA takes place using the viral RNA as a template. This process is started and stopped by the polymerase complex. Viral proteins are gathering in the cell as transcription occurs until the polymerase complex stops transcription and starts genome replication. Transcription of the viral RNA makes positive sense strands of RNA, which are then used as templates to make more negative sense viral RNA . Genome replication is halted before the viral particles can assemble to make a virion. Once the cell membrane is ready, new virions exit the host cell through budding.[27]
Vaccine
Henipaviruses have high mortality rates in mammalian hosts, both human and animal. Because of this, there is a need for immunization against HeV and NiV. Both diseases can be transmitted from animals to humans and can cause respiratory and neurological issues.[28] HeV and NiV appear as encephalitis or severe respiratory illnesses in human hosts. Animal hosts also experience respiratory distress and occasionally fever and neurological disease. Other vaccines for viruses in the Paramyxoviridae family use neutralizing antibodies directed to bind to the surface glycoproteins. A human vaccine for henipaviruses has still not been made, but would probably follow the same model as other vaccines for Paramyxoviridae family viruses.[29]
Causes of emergence
The emergence of henipaviruses parallels the emergence of other zoonotic viruses in recent decades. SARS coronavirus, Australian bat lyssavirus, Menangle virus, Marburg virus, COVID 19 and possibly Ebola viruses are also harboured by bats, and are capable of infecting a variety of other species. The emergence of each of these viruses has been linked to an increase in contact between bats and humans, sometimes involving an intermediate domestic animal host. The increased contact is driven both by human encroachment into the bats' territory (in the case of Nipah, specifically pigpens in said territory) and by movement of bats towards human populations due to changes in food distribution and loss of habitat.
There is evidence that habitat loss for flying foxes, both in South Asia and Australia (particularly along the east coast) as well as encroachment of human dwellings and agriculture into the remaining habitats, is creating greater overlap of human and flying fox distributions.
Taxonomy
| Genus | Species | Virus (Abbreviation) |
| Henipavirus | Cedar henipavirus | Cedar virus (CedV) |
| Ghanaian bat henipavirus | Kumasi virus (KV) | |
| Hendra henipavirus | Hendra virus (HeV) | |
| Mojiang henipavirus | Mòjiāng virus (MojV)[3] | |
| Nipah henipavirus | Nipah virus (NiV) | |
| Langya henipavirus | Langya virus (LayV)[6][31] |
See also
References
- ^ Rima, B; Balkema-Buschmann, A; Dundon, WG; Duprex, WP; Easton, A; Fouchier, R; Kurath, G; Lamb, R; Lee, B; Rota, P; Wang, L; ICTV Report Consortium (December 2019). "ICTV Virus Taxonomy Profile: Paramyxoviridae". The Journal of General Virology. 100 (12): 1593–1594. doi:10.1099/jgv.0.001328. PMC 7273325. PMID 31609197.
- ^ "ICTV Report Paramyxoviridae".
- ^ a b Wu, Zhiqiang; et al. (2014). "Novel Henipa-like Virus, Mojiang Paramyxovirus, in Rats, China, 2012". Emerging Infectious Diseases. 20 (6): 1064–1066. doi:10.3201/eid2006.131022. PMC 4036791. PMID 24865545.
- ^ Li, Y; Wang, J; Hickey, AC; Zhang, Y; Li, Y; Wu, Y; Zhang, Huajun; et al. (December 2008). "Antibodies to Nipah or Nipah-like viruses in bats, China [letter]". Emerging Infectious Diseases. 14 (12): 1974–6. doi:10.3201/eid1412.080359. PMC 2634619. PMID 19046545.
- ^ Cheng, Amy (10 August 2022). "New Langya virus that may have spilled over from animals infects dozens". The Washington Post.
- ^ a b Zhang, Xiao-Ai; et al. (2022). "A Zoonotic Henipavirus in Febrile Patients in China". The New England Journal of Medicine. 387 (5): 470–472. doi:10.1056/NEJMc2202705. PMID 35921459. S2CID 251315935.
- ^ Sawatsky (2008). "Hendra and Nipah Virus". Animal Viruses: Molecular Biology. Caister Academic Press. ISBN 978-1-904455-22-6.
- ^ "Nipah yet to be confirmed, 86 under observation: Shailaja". OnManorama. Retrieved 4 June 2019.
- ^ Drexler JF, Corman VM, Gloza-Rausch F, Seebens A, Annan A (2009). Markotter W (ed.). "Henipavirus RNA in African Bats". PLOS ONE. 4 (7): e6367. Bibcode:2009PLoSO...4.6367D. doi:10.1371/journal.pone.0006367. PMC 2712088. PMID 19636378.
- ^ Drexler JF, Corman VM; et al. (2012). "Bats host major mammalian paramyxoviruses". Nat Commun. 3: 796. Bibcode:2012NatCo...3..796D. doi:10.1038/ncomms1796. PMC 3343228. PMID 22531181.
- ^ "Federal Select Agent Program". www.selectagents.gov. 8 January 2021. Retrieved 15 January 2021.
- ^ Hyatt AD, Zaki SR, Goldsmith CS, Wise TG, Hengstberger SG (2001). "Ultrastructure of Hendra virus and Nipah virus within cultured cells and host animals". Microbes and Infection. 3 (4): 297–306. doi:10.1016/S1286-4579(01)01383-1. PMID 11334747.
- ^ Bonaparte, M; Dimitrov, A; Bossart, K (2005). "Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus". Proceedings of the National Academy of Sciences. 102 (30): 10652–7. Bibcode:2005PNAS..10210652B. doi:10.1073/pnas.0504887102. PMC 1169237. PMID 15998730.
- ^ Negrete OA, Levroney EL, Aguilar HC (2005). "EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus". Nature. 436 (7049): 401–5. Bibcode:2005Natur.436..401N. doi:10.1038/nature03838. PMID 16007075. S2CID 4367038.
- ^ Bowden, Thomas A.; Crispin, Max; Jones, E. Yvonne; Stuart, David I. (1 October 2010). "Shared paramyxoviral glycoprotein architecture is adapted for diverse attachment strategies". Biochemical Society Transactions. 38 (5): 1349–1355. doi:10.1042/BST0381349. PMC 3433257. PMID 20863312.
- ^ Bowden, Thomas A.; Crispin, Max; Harvey, David J.; Aricescu, A. Radu; Grimes, Jonathan M.; Jones, E. Yvonne; Stuart, David I. (1 December 2008). "Crystal Structure and Carbohydrate Analysis of Nipah Virus Attachment Glycoprotein: a Template for Antiviral and Vaccine Design". Journal of Virology. 82 (23): 11628–11636. doi:10.1128/JVI.01344-08. PMC 2583688. PMID 18815311.
- ^ Wang L, Harcourt BH, Yu M (2001). "Molecular biology of Hendra and Nipah viruses". Microbes and Infection. 3 (4): 279–87. doi:10.1016/S1286-4579(01)01381-8. PMID 11334745.
- ^ Halpin, Kim; Bankamp, Bettina; Harcourt, Brian H.; Bellini, William J.; Rota, Paul A.YR 2004. "Nipah virus conforms to the rule of six in a minigenome replication assay". Journal of General Virology. 85 (3): 701–707. doi:10.1099/vir.0.19685-0. ISSN 1465-2099.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Kolakofsky, D; Pelet, T; Garcin, D; Hausmann, S; Curran, J; Roux, L (February 1998). "Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited". Journal of Virology. 72 (2): 891–9. doi:10.1128/JVI.72.2.891-899.1998. PMC 124558. PMID 9444980.
- ^ Shaw, Megan L. (2009-12). "Henipaviruses Employ a Multifaceted Approach to Evade the Antiviral Interferon Response". Viruses. 1 (3): 1190–1203. doi:10.3390/v1031190. ISSN 1999-4915. PMC 3185527. PMID 21994589.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Lawrence, Philip; Escudero-Pérez, Beatriz (29 April 2022). "Henipavirus Immune Evasion and Pathogenesis Mechanisms: Lessons Learnt from Natural Infection and Animal Models". Viruses. 14 (5): 936. doi:10.3390/v14050936. ISSN 1999-4915. PMC 9146692. PMID 35632678.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Shaw, Megan L.; García-Sastre, Adolfo; Palese, Peter; Basler, Christopher F. (2004-06). "Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively". Journal of Virology. 78 (11): 5633–5641. doi:10.1128/JVI.78.11.5633-5641.2004. ISSN 0022-538X. PMC 415790. PMID 15140960.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Lo, Michael K.; Peeples, Mark E.; Bellini, William J.; Nichol, Stuart T.; Rota, Paul A.; Spiropoulou, Christina F. (19 October 2012). "Distinct and Overlapping Roles of Nipah Virus P Gene Products in Modulating the Human Endothelial Cell Antiviral Response". PLOS ONE. 7 (10): e47790. doi:10.1371/journal.pone.0047790. ISSN 1932-6203. PMC 3477106. PMID 23094089.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Park, Arnold; Yun, Tatyana; Vigant, Frederic; Pernet, Olivier; Won, Sohui T.; Dawes, Brian E.; Bartkowski, Wojciech; Freiberg, Alexander N.; Lee, Benhur (20 May 2016). "Nipah Virus C Protein Recruits Tsg101 to Promote the Efficient Release of Virus in an ESCRT-Dependent Pathway". PLOS Pathogens. 12 (5): e1005659. doi:10.1371/journal.ppat.1005659. ISSN 1553-7374.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Bonaparte, Matthew I.; Dimitrov, Antony S.; Bossart, Katharine N.; Crameri, Gary; Mungall, Bruce A.; Bishop, Kimberly A.; Choudhry, Vidita; Dimitrov, Dimiter S.; Wang, Lin-Fa; Eaton, Bryan T.; Broder, Christopher C. (5 July 2005). "Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus". Proceedings of the National Academy of Sciences. 102 (30): 10652–10657. Bibcode:2005PNAS..10210652B. doi:10.1073/pnas.0504887102. ISSN 0027-8424. PMC 1169237. PMID 15998730.
- ^ Zuckerman, Arie J. (10 June 1996). "Fields virology, 3rd edn. (two vol. set): Edited by B.N. Fields, D.M. Knipe, P.M. Howley, R.M. Chanock, J.L. Melnick, T.P. Monath, B. Roizman and S.E. Straus, Lippincott-Raven, Philadelphia, P". FEBS Letters. 388 (1): 88. doi:10.1016/0014-5793(96)88179-8.
- ^ Rota, Paul A.; Lo, Michael K. (2012), Lee, Benhur; Rota, Paul A. (eds.), "Molecular Virology of the Henipaviruses", Henipavirus, vol. 359, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 41–58, doi:10.1007/82_2012_211, ISBN 978-3-642-29818-9, PMID 22552699, retrieved 4 December 2022
- ^ Wong, K. Thong; Grosjean, Isabelle; Brisson, Christine; Blanquier, Barissa; Fevre-Montange, Michelle; Bernard, Arlette; Loth, Philippe; Georges-Courbot, Marie-Claude; Chevallier, Michelle; Akaoka, Hideo; Marianneau, Philippe; Lam, Sai Kit; Wild, T. Fabian; Deubel, Vincent (November 2003). "A Golden Hamster Model for Human Acute Nipah Virus Infection". The American Journal of Pathology. 163 (5): 2127–2137. doi:10.1016/s0002-9440(10)63569-9. ISSN 0002-9440. PMC 1892425. PMID 14578210.
- ^ Broder, Christopher C.; Geisbert, Thomas W.; Xu, Kai; Nikolov, Dimitar B.; Wang, Lin-Fa; Middleton, Deborah; Pallister, Jackie; Bossart, Katharine N. (2012), Lee, Benhur; Rota, Paul A. (eds.), "Immunization Strategies Against Henipaviruses", Henipavirus, vol. 359, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 197–223, doi:10.1007/82_2012_213, ISBN 978-3-642-29818-9, PMC 4465348, PMID 22481140
- ^ Amarasinghe, Gaya K.; Bào, Yīmíng; Basler, Christopher F.; Bavari, Sina; Beer, Martin; Bejerman, Nicolás; Blasdell, Kim R.; Bochnowski, Alisa; Briese, Thomas (7 April 2017). "Taxonomy of the order Mononegavirales: update 2017". Archives of Virology. 162 (8): 2493–2504. doi:10.1007/s00705-017-3311-7. ISSN 1432-8798. PMC 5831667. PMID 28389807.
- ^ "Zoonotic Langya virus found in China, CDC says - Taipei Times". 9 August 2022.
External links
- ICTV Report: Paramyxoviridae
- Disease card
- ViralZone: Henipavirus
- Henipavirus – Henipavirus Ecology Research Group (HERG) INFO
- Virus Pathogen Database and Analysis Resource (ViPR): Paramyxoviridae
