Drug-eluting stent: Difference between revisions
Altered template type. Add: page, issue, isbn, pages, chapter, chapter-url, vauthors, s2cid, journal, date, title, doi, pmc, pmid, volume, authors 1-20. Removed or converted URL. Changed bare reference to CS1/2. | Use this tool. Report bugs. | #UCB_Gadget |
|||
| Line 155: | Line 155: | ||
Though it occurred less frequently than with balloon angioplasty or other techniques, stents nonetheless remained vulnerable to restenosis, caused almost exclusively by neointimal tissue growth (tissue formation in the inner 'tube' structure of the artery). To address this issue, developers of drug-eluting stents used the devices themselves as a tool for delivering medication directly to the arterial wall. While initial efforts were unsuccessful, the release (elution) of drugs with certain specific physicochemical properties from the stent was shown in 2001 to achieve high concentrations of the drug locally, directly at the target lesion, with minimal systemic side effects.<ref name="pmid11479260">{{cite journal | vauthors = Hwang CW, Wu D, Edelman ER | title = Physiological transport forces govern drug distribution for stent-based delivery | journal = Circulation | volume = 104 | issue = 5 | pages = 600–605 | date = July 2001 | pmid = 11479260 | doi = 10.1161/hc3101.092214 | doi-access = free }}</ref> As currently used in clinical practice, "drug-eluting" stents refers to metal stents that elute a drug designed to limit the growth of neointimal scar tissue, thus reducing the likelihood of stent [[restenosis]].<ref name="Ellis-2006">{{cite book |vauthors=Ellis SG, Holme DR |url=https://books.google.com/books?id=uqft1t92S88C&q=stent+restenosis&pg=PA358 |title=Strategic Approaches in Coronary Intervention |date=2006 |publisher=Lippincott Williams & Wilkins |isbn=978-0-7817-4294-8 |access-date=13 May 2015 |archive-date=20 October 2023 |archive-url=https://web.archive.org/web/20231020234539/https://books.google.com/books?id=uqft1t92S88C&q=stent+restenosis&pg=PA358 |url-status=live }}</ref> |
Though it occurred less frequently than with balloon angioplasty or other techniques, stents nonetheless remained vulnerable to restenosis, caused almost exclusively by neointimal tissue growth (tissue formation in the inner 'tube' structure of the artery). To address this issue, developers of drug-eluting stents used the devices themselves as a tool for delivering medication directly to the arterial wall. While initial efforts were unsuccessful, the release (elution) of drugs with certain specific physicochemical properties from the stent was shown in 2001 to achieve high concentrations of the drug locally, directly at the target lesion, with minimal systemic side effects.<ref name="pmid11479260">{{cite journal | vauthors = Hwang CW, Wu D, Edelman ER | title = Physiological transport forces govern drug distribution for stent-based delivery | journal = Circulation | volume = 104 | issue = 5 | pages = 600–605 | date = July 2001 | pmid = 11479260 | doi = 10.1161/hc3101.092214 | doi-access = free }}</ref> As currently used in clinical practice, "drug-eluting" stents refers to metal stents that elute a drug designed to limit the growth of neointimal scar tissue, thus reducing the likelihood of stent [[restenosis]].<ref name="Ellis-2006">{{cite book |vauthors=Ellis SG, Holme DR |url=https://books.google.com/books?id=uqft1t92S88C&q=stent+restenosis&pg=PA358 |title=Strategic Approaches in Coronary Intervention |date=2006 |publisher=Lippincott Williams & Wilkins |isbn=978-0-7817-4294-8 |access-date=13 May 2015 |archive-date=20 October 2023 |archive-url=https://web.archive.org/web/20231020234539/https://books.google.com/books?id=uqft1t92S88C&q=stent+restenosis&pg=PA358 |url-status=live }}</ref> |
||
The first type of DES to be approved by the [[European Medicines Agency]] (EMA) and the [[US Food and Drug Administration]] (FDA) were sirolimus-eluting stents (SES), which release a natural product called [[sirolimus]],<ref name="European Medicines Agency-2007">{{cite web|url=https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-development-medicinal-substances-contained-drug-eluting-medicinal-substance-eluting-coronary-stents_en.pdf|publisher=European Medicines Agency|title=Guideline on the development of medicinal substances contained in drug-eluting (medicinal substance-eluting) coronary stents|date=22 March 2007|location=London|access-date=8 February 2024|archive-date=8 February 2024|archive-url=https://web.archive.org/web/20240208135121/https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-development-medicinal-substances-contained-drug-eluting-medicinal-substance-eluting-coronary-stents_en.pdf|url-status=live}}</ref> an immunosuppressant drug.<ref name="pmid16459533">{{cite journal |vauthors=Mukherjee T, Shah BV |title=Sirolimus: a new immunosuppressant |journal=J Assoc Physicians India |volume=53 |issue= |pages=885–90 |date=October 2005 |pmid=16459533}}</ref> SES were shown to reduce the need for repeat procedures and improve the outcomes of patients with coronary artery disease.<ref name="sirolimus-1">{{cite journal|url=https://clinicaltrials.gov/ct2/show/NCT00258596|title=Sirolimus-Eluting Stents for Chronic Total Coronary Occlusions - Full Text View - ClinicalTrials.gov|website=clinicaltrials.gov|date=5 March 2007 |access-date=8 February 2024|archive-date=13 April 2021|archive-url=https://web.archive.org/web/20210413155108/https://www.clinicaltrials.gov/ct2/show/NCT00258596|url-status=live}}</ref><ref name="sirolimus-0">{{cite journal|url=https://clinicaltrials.gov/ct2/show/NCT01443104|title=Sirolimus-eluting Stents With Biodegradable Polymer Versus an Everolimus-eluting Stents - Full Text View - ClinicalTrials.gov|website=clinicaltrials.gov|date=16 October 2018 |access-date=8 February 2024|archive-date=15 May 2021|archive-url=https://web.archive.org/web/20210515010204/https://clinicaltrials.gov/ct2/show/NCT01443104|url-status=live}}</ref><ref name="trials-0">{{cite journal|url=https://clinicaltrials.gov/ct2/show/NCT02448524|title=Clinical Trial on the Efficacy and Safety of Sirolimus-Eluting Stent (MiStent® System) - Full Text View - ClinicalTrials.gov|website=clinicaltrials.gov|date=3 September 2020 |access-date=8 February 2024|archive-date=7 April 2021|archive-url=https://web.archive.org/web/20210407121623/https://www.clinicaltrials.gov/ct2/show/NCT02448524|url-status=live}}</ref> The sirolimus-eluting Cypher stent received CE mark approval in Europe in 2002, and then underwent a larger trial to demonstrate its safety and effectiveness for the US market.<ref name="trials-1">{{cite journal|url=http://www.nejm.org/doi/10.1056/NEJMoa067731|title=Stent Thrombosis in Randomized Clinical Trials of Drug-Eluting Stents|first1=Laura|last1=Mauri|first2=Wen-hua|last2=Hsieh|first3=Joseph M.|last3=Massaro|first4=Kalon K.L.|last4=Ho|first5=Ralph|last5=D'Agostino|first6=Donald E.|last6=Cutlip|date=8 March 2007|journal=New England Journal of Medicine|volume=356|issue=10|pages=1020–1029|via=CrossRef|doi=10.1056/NEJMoa067731|pmid=17296821|access-date=8 February 2024|archive-date=3 May 2023|archive-url=https://web.archive.org/web/20230503095147/https://www.nejm.org/doi/10.1056/NEJMoa067731|url-status=live}}</ref><ref name="trials-2">{{cite web|url=https://clinicaltrials.gov/ct2/show/NCT00297804|title=A Randomized, Double-blind Study to Assess Paclitaxel-eluting Stents in Treatment of Longer Lesions - Full Text View - ClinicalTrials.gov|website=clinicaltrials.gov|access-date=8 February 2024|archive-date=6 April 2021|archive-url=https://web.archive.org/web/20210406102936/https://clinicaltrials.gov/ct2/show/NCT00297804|url-status=live}}</ref><ref name="trials-3">{{cite journal|url=https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.105.552190|title=Clinical Efficacy of Polymer-Based Paclitaxel-Eluting Stents in the Treatment of Complex, Long Coronary Artery Lesions From a Multicenter, Randomized Trial: Support for the Use of Drug-Eluting Stents in Contemporary Clinical Practice|first1=Keith D.|last1=Dawkins|first2=Eberhard|last2=Grube|first3=Giulio|last3=Guagliumi|first4=Adrian P.|last4=Banning|first5=Krzysztof|last5=Zmudka|first6=Antonio|last6=Colombo|first7=Leif|last7=Thuesen|first8=Karl|last8=Hauptman|first9=Jean|last9=Marco|first10=William|last10=Wijns|first11=Jeffrey J.|last11=Popma|first12=Joerg|last12=Koglin|first13=Mary E.|last13=Russell|date=22 November 2005|journal=Circulation|volume=112|issue=21|pages=3306–3313|via=CrossRef|doi=10.1161/CIRCULATIONAHA.105.552190|pmid=16286586|access-date=8 February 2024|archive-date=8 February 2024|archive-url=https://web.archive.org/web/20240208142716/https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.105.552190|url-status=live}}</ref> The trial, published in 2003, enrolled 1058 patients with more complex lesions and confirmed the superiority of SES over bare metal stents in terms of angiographic and clinical outcomes.<ref name="eluvia-trials">{{cite web|url=https://www.bostonscientific.com/en-EU/medical-specialties/vascular-surgery/drug-eluting-therapies1/eluvia/eluvia-clinical-trials.html|title=Clinical Trial Results - Eluvia Drug-Eluting Stent|website=www.bostonscientific.com|access-date=8 February 2024|archive-date=8 February 2024|archive-url=https://web.archive.org/web/20240208142715/https://www.bostonscientific.com/en-EU/medical-specialties/vascular-surgery/drug-eluting-therapies1/eluvia/eluvia-clinical-trials.html|url-status=live}}</ref><ref name="trials-4">{{cite journal|url=https://doi.org/10.1001/jama.299.4.409|title=Comparison of Paclitaxel- and Sirolimus-Eluting Stents in Everyday Clinical PracticeThe SORT OUT II Randomized Trial|first1=Anders M.|last1=Galløe|first2=Leif|last2=Thuesen|first3=Henning|last3=Kelbæk|first4=Per|last4=Thayssen|first5=Klaus|last5=Rasmussen|first6=Peter R.|last6=Hansen|first7=Niels|last7=Bligaard|first8=Kari|last8=Saunamäki|first9=Anders|last9=Junker|first10=Jens|last10=Aarøe|first11=Ulrik|last11=Abildgaard|first12=Jan|last12=Ravkilde|first13=Thomas|last13=Engstrøm|first14=Jan S.|last14=Jensen|first15=Henning R.|last15=Andersen|first16=Hans E.|last16=Bøtker|first17=Søren|last17=Galatius|first18=Steen D.|last18=Kristensen|first19=Jan K.|last19=Madsen|first20=Lars R.|last20=Krusell|first21=Steen Z.|last21=Abildstrøm|first22=Ghita B.|last22=Stephansen|first23=Jens F.|last23=Lassen|first24=for the|last24=SORT OUT II Investigators|date=30 January 2008|journal=JAMA|volume=299|issue=4|pages=409–416|via=Silverchair|doi=10.1001/jama.299.4.409|pmid=18230778|url-access=subscription|access-date=8 February 2024|archive-date=24 February 2024|archive-url=https://web.archive.org/web/20240224233743/https://jamanetwork.com/journals/jama/fullarticle/1149166|url-status=live}}</ref><ref name="trials-5">{{cite journal|title=Clinical outcomes in 2481 unselected real-world patients treated with a polymer-free sirolimus-eluting stent: 3 years results from the NANO multicenter Registry|first1=Yi|last1=Dai|first2=Rutao|last2=Wang|first3=Fengying|last3=Chen|first4=Yaojun|last4=Zhang|first5=Yi|last5=Liu|first6=He|last6=Huang|first7=Ping|last7=Yang|first8=Ruining|last8=Zhang|first9=Bo|last9=Zheng|first10=Chao|last10=Gao|first11=Yundai|last11=Chen|first12=Ling|last12=Tao|date=12 November 2021|journal=BMC Cardiovascular Disorders|volume=21|issue=1|pages=537|doi=10.1186/s12872-021-02356-0|doi-access=free |pmid=34772347|pmc=8588634}}</ref><ref name="r5">{{cite journal|url=https://www.thelancet.com/pdfs/journals/eclinm/PIIS2589-5370%2823%2900481-9.pdf|doi=10.1016/j.eclinm.2023.102304|journal=eClinicalMedicine|title=First randomised controlled trial comparing the sirolimus-eluting bioadaptor with the zotarolimus-eluting drug-eluting stent in patients with de novo coronary artery lesions: 12-month clinical and imaging data from the multi-centre, international, BIODAPTOR-RCT|date=November 2023}}</ref> Based on these results, the Cypher stent received FDA approval and was released in the US in 2003.<ref name="pmid16452560" /> The FDA approval process for DES involves submitting an investigational device exemption (IDE) application to conduct clinical trials under 21 CFR Part 812, and then a premarket approval (PMA) application to obtain marketing authorization under 21 CFR Part 8144. The FDA assigns the primary review responsibility to the Center for Devices and Radiological Health (CDRH), but also consults with the Center for Drug Evaluation and Research (CDER) for the drug component of the combination product. |
The first type of DES to be approved by the [[European Medicines Agency]] (EMA) and the [[US Food and Drug Administration]] (FDA) were sirolimus-eluting stents (SES), which release a natural product called [[sirolimus]],<ref name="European Medicines Agency-2007">{{cite web|url=https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-development-medicinal-substances-contained-drug-eluting-medicinal-substance-eluting-coronary-stents_en.pdf|publisher=European Medicines Agency|title=Guideline on the development of medicinal substances contained in drug-eluting (medicinal substance-eluting) coronary stents|date=22 March 2007|location=London|access-date=8 February 2024|archive-date=8 February 2024|archive-url=https://web.archive.org/web/20240208135121/https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-development-medicinal-substances-contained-drug-eluting-medicinal-substance-eluting-coronary-stents_en.pdf|url-status=live}}</ref> an immunosuppressant drug.<ref name="pmid16459533">{{cite journal |vauthors=Mukherjee T, Shah BV |title=Sirolimus: a new immunosuppressant |journal=J Assoc Physicians India |volume=53 |issue= |pages=885–90 |date=October 2005 |pmid=16459533}}</ref> SES were shown to reduce the need for repeat procedures and improve the outcomes of patients with coronary artery disease.<ref name="sirolimus-1">{{cite journal|url=https://clinicaltrials.gov/ct2/show/NCT00258596|title=Sirolimus-Eluting Stents for Chronic Total Coronary Occlusions - Full Text View - ClinicalTrials.gov|website=clinicaltrials.gov|date=5 March 2007 |access-date=8 February 2024|archive-date=13 April 2021|archive-url=https://web.archive.org/web/20210413155108/https://www.clinicaltrials.gov/ct2/show/NCT00258596|url-status=live}}</ref><ref name="sirolimus-0">{{cite journal|url=https://clinicaltrials.gov/ct2/show/NCT01443104|title=Sirolimus-eluting Stents With Biodegradable Polymer Versus an Everolimus-eluting Stents - Full Text View - ClinicalTrials.gov|website=clinicaltrials.gov|date=16 October 2018 |access-date=8 February 2024|archive-date=15 May 2021|archive-url=https://web.archive.org/web/20210515010204/https://clinicaltrials.gov/ct2/show/NCT01443104|url-status=live}}</ref><ref name="trials-0">{{cite journal|url=https://clinicaltrials.gov/ct2/show/NCT02448524|title=Clinical Trial on the Efficacy and Safety of Sirolimus-Eluting Stent (MiStent® System) - Full Text View - ClinicalTrials.gov|website=clinicaltrials.gov|date=3 September 2020 |access-date=8 February 2024|archive-date=7 April 2021|archive-url=https://web.archive.org/web/20210407121623/https://www.clinicaltrials.gov/ct2/show/NCT02448524|url-status=live}}</ref> The sirolimus-eluting Cypher stent received CE mark approval in Europe in 2002, and then underwent a larger trial to demonstrate its safety and effectiveness for the US market.<ref name="trials-1">{{cite journal|url=http://www.nejm.org/doi/10.1056/NEJMoa067731|title=Stent Thrombosis in Randomized Clinical Trials of Drug-Eluting Stents|first1=Laura|last1=Mauri|first2=Wen-hua|last2=Hsieh|first3=Joseph M.|last3=Massaro|first4=Kalon K.L.|last4=Ho|first5=Ralph|last5=D'Agostino|first6=Donald E.|last6=Cutlip|date=8 March 2007|journal=New England Journal of Medicine|volume=356|issue=10|pages=1020–1029|via=CrossRef|doi=10.1056/NEJMoa067731|pmid=17296821|access-date=8 February 2024|archive-date=3 May 2023|archive-url=https://web.archive.org/web/20230503095147/https://www.nejm.org/doi/10.1056/NEJMoa067731|url-status=live}}</ref><ref name="trials-2">{{cite web|url=https://clinicaltrials.gov/ct2/show/NCT00297804|title=A Randomized, Double-blind Study to Assess Paclitaxel-eluting Stents in Treatment of Longer Lesions - Full Text View - ClinicalTrials.gov|website=clinicaltrials.gov|access-date=8 February 2024|archive-date=6 April 2021|archive-url=https://web.archive.org/web/20210406102936/https://clinicaltrials.gov/ct2/show/NCT00297804|url-status=live}}</ref><ref name="trials-3">{{cite journal|url=https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.105.552190|title=Clinical Efficacy of Polymer-Based Paclitaxel-Eluting Stents in the Treatment of Complex, Long Coronary Artery Lesions From a Multicenter, Randomized Trial: Support for the Use of Drug-Eluting Stents in Contemporary Clinical Practice|first1=Keith D.|last1=Dawkins|first2=Eberhard|last2=Grube|first3=Giulio|last3=Guagliumi|first4=Adrian P.|last4=Banning|first5=Krzysztof|last5=Zmudka|first6=Antonio|last6=Colombo|first7=Leif|last7=Thuesen|first8=Karl|last8=Hauptman|first9=Jean|last9=Marco|first10=William|last10=Wijns|first11=Jeffrey J.|last11=Popma|first12=Joerg|last12=Koglin|first13=Mary E.|last13=Russell|date=22 November 2005|journal=Circulation|volume=112|issue=21|pages=3306–3313|via=CrossRef|doi=10.1161/CIRCULATIONAHA.105.552190|pmid=16286586|access-date=8 February 2024|archive-date=8 February 2024|archive-url=https://web.archive.org/web/20240208142716/https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.105.552190|url-status=live}}</ref> The trial, published in 2003, enrolled 1058 patients with more complex lesions and confirmed the superiority of SES over bare metal stents in terms of angiographic and clinical outcomes.<ref name="eluvia-trials">{{cite web|url=https://www.bostonscientific.com/en-EU/medical-specialties/vascular-surgery/drug-eluting-therapies1/eluvia/eluvia-clinical-trials.html|title=Clinical Trial Results - Eluvia Drug-Eluting Stent|website=www.bostonscientific.com|access-date=8 February 2024|archive-date=8 February 2024|archive-url=https://web.archive.org/web/20240208142715/https://www.bostonscientific.com/en-EU/medical-specialties/vascular-surgery/drug-eluting-therapies1/eluvia/eluvia-clinical-trials.html|url-status=live}}</ref><ref name="trials-4">{{cite journal|url=https://doi.org/10.1001/jama.299.4.409|title=Comparison of Paclitaxel- and Sirolimus-Eluting Stents in Everyday Clinical PracticeThe SORT OUT II Randomized Trial|first1=Anders M.|last1=Galløe|first2=Leif|last2=Thuesen|first3=Henning|last3=Kelbæk|first4=Per|last4=Thayssen|first5=Klaus|last5=Rasmussen|first6=Peter R.|last6=Hansen|first7=Niels|last7=Bligaard|first8=Kari|last8=Saunamäki|first9=Anders|last9=Junker|first10=Jens|last10=Aarøe|first11=Ulrik|last11=Abildgaard|first12=Jan|last12=Ravkilde|first13=Thomas|last13=Engstrøm|first14=Jan S.|last14=Jensen|first15=Henning R.|last15=Andersen|first16=Hans E.|last16=Bøtker|first17=Søren|last17=Galatius|first18=Steen D.|last18=Kristensen|first19=Jan K.|last19=Madsen|first20=Lars R.|last20=Krusell|first21=Steen Z.|last21=Abildstrøm|first22=Ghita B.|last22=Stephansen|first23=Jens F.|last23=Lassen|first24=for the|last24=SORT OUT II Investigators|date=30 January 2008|journal=JAMA|volume=299|issue=4|pages=409–416|via=Silverchair|doi=10.1001/jama.299.4.409|pmid=18230778|url-access=subscription|access-date=8 February 2024|archive-date=24 February 2024|archive-url=https://web.archive.org/web/20240224233743/https://jamanetwork.com/journals/jama/fullarticle/1149166|url-status=live}}</ref><ref name="trials-5">{{cite journal|title=Clinical outcomes in 2481 unselected real-world patients treated with a polymer-free sirolimus-eluting stent: 3 years results from the NANO multicenter Registry|first1=Yi|last1=Dai|first2=Rutao|last2=Wang|first3=Fengying|last3=Chen|first4=Yaojun|last4=Zhang|first5=Yi|last5=Liu|first6=He|last6=Huang|first7=Ping|last7=Yang|first8=Ruining|last8=Zhang|first9=Bo|last9=Zheng|first10=Chao|last10=Gao|first11=Yundai|last11=Chen|first12=Ling|last12=Tao|date=12 November 2021|journal=BMC Cardiovascular Disorders|volume=21|issue=1|pages=537|doi=10.1186/s12872-021-02356-0|doi-access=free |pmid=34772347|pmc=8588634}}</ref><ref name="r5">{{cite journal|url=https://www.thelancet.com/pdfs/journals/eclinm/PIIS2589-5370%2823%2900481-9.pdf|doi=10.1016/j.eclinm.2023.102304|journal=eClinicalMedicine|title=First randomised controlled trial comparing the sirolimus-eluting bioadaptor with the zotarolimus-eluting drug-eluting stent in patients with de novo coronary artery lesions: 12-month clinical and imaging data from the multi-centre, international, BIODAPTOR-RCT|date=November 2023 |author20=BIOADAPTOR-RCT Collaborators |volume=65 |pmid=38106564 |pmc=10725075 | vauthors = Saito S, Bennett J, Nef HM, Webster M, Namiki A, Takahashi A, Kakuta T, Yamazaki S, Shibata Y, Scott D, Vrolix M, Menon M, Möllmann H, Werner N, Neylon A, Mehmedbegovic Z, Smits PC, Morice M, Verheye S }}</ref> Based on these results, the Cypher stent received FDA approval and was released in the US in 2003.<ref name="pmid16452560" /> The FDA approval process for DES involves submitting an investigational device exemption (IDE) application to conduct clinical trials under 21 CFR Part 812, and then a premarket approval (PMA) application to obtain marketing authorization under 21 CFR Part 8144. The FDA assigns the primary review responsibility to the Center for Devices and Radiological Health (CDRH), but also consults with the Center for Drug Evaluation and Research (CDER) for the drug component of the combination product. |
||
The second type of DES to be approved by the EMA and the FDA were paclitaxel-eluting stents (PES), which release another natural product called [[paclitaxel]]. PES also reduced the need for repeat procedures and improved the outcomes of patients with different types of lesions and risk factors. The paclitaxel-eluting Taxus stent received FDA approval and was launched in the US in 2004,<ref name="fda-2004">{{cite journal|url=https://www.fda.gov/combination-products/jurisdictional-updates/jurisdictional-update-drug-eluting-cardiovascular-stents|title=Jurisdictional Update: Drug-Eluting Cardiovascular Stents|first=Office of the|last=Commissioner|date=3 November 2018|journal=FDA|via=www.fda.gov|access-date=8 February 2024|archive-date=8 December 2023|archive-url=https://web.archive.org/web/20231208172253/https://www.fda.gov/combination-products/jurisdictional-updates/jurisdictional-update-drug-eluting-cardiovascular-stents|url-status=live}}</ref> after a series of trials that compared it with a bare metal stent in various settings. The trials showed a significant reduction in target lesion revascularization and major adverse cardiac events with the Taxus stent at 9 and 12 months. Both SES and PES use natural products as the active agents to prevent the recurrence of blockages in the arteries.<ref name="pmid29678389"/> These DES have changed the practice of interventional cardiology and have become the preferred treatment for many patients with coronary artery disease.<ref name="pmid29678389">{{cite journal | vauthors = Uhrin P, Wang D, Mocan A, Waltenberger B, Breuss JM, Tewari D, Mihaly-Bison J, Huminiecki Ł, Starzyński RR, Tzvetkov NT, Horbańczuk J, Atanasov AG | title = Vascular smooth muscle cell proliferation as a therapeutic target. Part 2: Natural products inhibiting proliferation | journal = Biotechnology Advances | volume = 36 | issue = 6 | pages = 1608–1621 | date = November 2018 | pmid = 29678389 | doi = 10.1016/j.biotechadv.2018.04.002 | s2cid = 5027489 }}</ref><ref name="new-types-lancet">{{cite web|url=https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736%2819%2930474-X.pdf|title=Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials - The Lancet|access-date=8 February 2024|archive-date=8 February 2024|archive-url=https://web.archive.org/web/20240208135547/https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(19)30474-X.pdf|url-status=live}}</ref><ref name="pmid37733656">{{cite journal|title=Meta-analysis of outcomes from drug-eluting stent implantation in femoropopliteal arteries|first1=Mingxuan|last1=Li|first2=Haixia|last2=Tu|first3=Yu|last3=Yan|first4=Zhen|last4=Guo|first5=Haitao|last5=Zhu|first6=Jiangliang|last6=Niu|first7=Mengchen|last7=Yin|date=21 September 2023|journal=PLOS ONE|volume=18|issue=9|pages=e0291466|doi=10.1371/journal.pone.0291466|doi-access=free |pmid=37733656|pmc=10513203|bibcode=2023PLoSO..1891466L }}</ref> |
The second type of DES to be approved by the EMA and the FDA were paclitaxel-eluting stents (PES), which release another natural product called [[paclitaxel]]. PES also reduced the need for repeat procedures and improved the outcomes of patients with different types of lesions and risk factors. The paclitaxel-eluting Taxus stent received FDA approval and was launched in the US in 2004,<ref name="fda-2004">{{cite journal|url=https://www.fda.gov/combination-products/jurisdictional-updates/jurisdictional-update-drug-eluting-cardiovascular-stents|title=Jurisdictional Update: Drug-Eluting Cardiovascular Stents|first=Office of the|last=Commissioner|date=3 November 2018|journal=FDA|via=www.fda.gov|access-date=8 February 2024|archive-date=8 December 2023|archive-url=https://web.archive.org/web/20231208172253/https://www.fda.gov/combination-products/jurisdictional-updates/jurisdictional-update-drug-eluting-cardiovascular-stents|url-status=live}}</ref> after a series of trials that compared it with a bare metal stent in various settings. The trials showed a significant reduction in target lesion revascularization and major adverse cardiac events with the Taxus stent at 9 and 12 months. Both SES and PES use natural products as the active agents to prevent the recurrence of blockages in the arteries.<ref name="pmid29678389"/> These DES have changed the practice of interventional cardiology and have become the preferred treatment for many patients with coronary artery disease.<ref name="pmid29678389">{{cite journal | vauthors = Uhrin P, Wang D, Mocan A, Waltenberger B, Breuss JM, Tewari D, Mihaly-Bison J, Huminiecki Ł, Starzyński RR, Tzvetkov NT, Horbańczuk J, Atanasov AG | title = Vascular smooth muscle cell proliferation as a therapeutic target. Part 2: Natural products inhibiting proliferation | journal = Biotechnology Advances | volume = 36 | issue = 6 | pages = 1608–1621 | date = November 2018 | pmid = 29678389 | doi = 10.1016/j.biotechadv.2018.04.002 | s2cid = 5027489 }}</ref><ref name="new-types-lancet">{{cite web|url=https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736%2819%2930474-X.pdf|title=Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials - The Lancet|access-date=8 February 2024|archive-date=8 February 2024|archive-url=https://web.archive.org/web/20240208135547/https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(19)30474-X.pdf|url-status=live}}</ref><ref name="pmid37733656">{{cite journal|title=Meta-analysis of outcomes from drug-eluting stent implantation in femoropopliteal arteries|first1=Mingxuan|last1=Li|first2=Haixia|last2=Tu|first3=Yu|last3=Yan|first4=Zhen|last4=Guo|first5=Haitao|last5=Zhu|first6=Jiangliang|last6=Niu|first7=Mengchen|last7=Yin|date=21 September 2023|journal=PLOS ONE|volume=18|issue=9|pages=e0291466|doi=10.1371/journal.pone.0291466|doi-access=free |pmid=37733656|pmc=10513203|bibcode=2023PLoSO..1891466L }}</ref> |
||
The concept of using absorbable (also called biodegradable, bioabsorbable or bioresorbable)<ref name="br">https://link.springer.com/article/10.1007/s40735-019-0216-x</ref> materials in stents was first reported in 1878 by Huse who used magnesium wires as ligatures to halt the bleeding in vessels of three patients. Despite extensive search, the full name of this pioneer in the field remains elusive. <ref name="br"/><ref>https://link.springer.com/chapter/10.1007/978-1-4614-3942-4_5</ref> In 20th century, a resorbable stent tested in humans was developed by the Igaki Medical Planning Company in Japan and was constructed from poly-L-lactic acid (a form of [[polylactic acid]]); they published their initial results in 2000.<ref name="pmid20031723">{{cite journal | vauthors = Ormiston JA, Serruys PW | title = Bioabsorbable coronary stents | journal = Circulation. Cardiovascular Interventions | volume = 2 | issue = 3 | pages = 255–260 | date = June 2009 | pmid = 20031723 | doi = 10.1161/CIRCINTERVENTIONS.109.859173 | doi-access = free }}</ref> The German company [[Biotronik]] developed a [[magnesium]] absorbable (bioresorbable) stent and published clinical results in 2007.<ref name="pmid20031723" /> |
The concept of using absorbable (also called biodegradable, bioabsorbable or bioresorbable)<ref name="br">{{cite journal | url=https://link.springer.com/article/10.1007/s40735-019-0216-x | doi=10.1007/s40735-019-0216-x | title=Magnesium-Based Bioresorbable Stent Materials: Review of Reviews | date=2019 | journal=Journal of Bio- and Tribo-Corrosion | volume=5 | s2cid=80811999 | vauthors = Aljihmani L, Alic L, Boudjemline Y, Hijazi ZM, Mansoor B, Serpedin E, Qaraqe K }}</ref> materials in stents was first reported in 1878 by Huse who used magnesium wires as ligatures to halt the bleeding in vessels of three patients. Despite extensive search, the full name of this pioneer in the field remains elusive. <ref name="br"/><ref>{{cite book | chapter-url=https://link.springer.com/chapter/10.1007/978-1-4614-3942-4_5 | doi=10.1007/978-1-4614-3942-4_5 | chapter=Biodegradable Metals | title=Degradation of Implant Materials | date=2012 | pages=93–109 | isbn=978-1-4614-3941-7 | vauthors = Witte F, Eliezer A }}</ref> In 20th century, a resorbable stent tested in humans was developed by the Igaki Medical Planning Company in Japan and was constructed from poly-L-lactic acid (a form of [[polylactic acid]]); they published their initial results in 2000.<ref name="pmid20031723">{{cite journal | vauthors = Ormiston JA, Serruys PW | title = Bioabsorbable coronary stents | journal = Circulation. Cardiovascular Interventions | volume = 2 | issue = 3 | pages = 255–260 | date = June 2009 | pmid = 20031723 | doi = 10.1161/CIRCINTERVENTIONS.109.859173 | doi-access = free }}</ref> The German company [[Biotronik]] developed a [[magnesium]] absorbable (bioresorbable) stent and published clinical results in 2007.<ref name="pmid20031723" /> |
||
The first company to bring a bioresorbable stent to market was [[Abbott Laboratories|Abbott Vascular]] which received European marketing approval in September 2012; the second was Elixir which received its CE mark in May 2013.<ref name="pmid26113479">{{cite journal | vauthors = Charpentier E, Barna A, Guillevin L, Juliard JM | title = Fully bioresorbable drug-eluting coronary scaffolds: A review | journal = Archives of Cardiovascular Diseases | volume = 108 | issue = 6–7 | pages = 385–397 | year = 2015 | pmid = 26113479 | doi = 10.1016/j.acvd.2015.03.009 | doi-access = }}</ref><ref name="www.fiercemedicaldevices.com">Damian Garde for Fierce Medical Devices. 22 May 2013 [http://www.fiercemedicaldevices.com/story/boston-scientific-elixir-make-waves-europcr-2013/2013-05-22 Boston Scientific, Elixir make waves at EuroPCR 2013] {{Webarchive|url=https://web.archive.org/web/20160116032410/http://www.fiercemedicaldevices.com/story/boston-scientific-elixir-make-waves-europcr-2013/2013-05-22 |date=16 January 2016 }}</ref><ref name="bioresorbable-2">{{cite journal|title=Bioresorbable vascular stents and drug-eluting stents in treatment of coronary heart disease: a meta-analysis|first1=Le|last1=Ni|first2=Hao|last2=Chen|first3=Zhurong|last3=Luo|first4=Yunqiang|last4=Yu|date=28 January 2020|journal=Journal of Cardiothoracic Surgery|volume=15|issue=1|pages=26|doi=10.1186/s13019-020-1041-5|doi-access=free |pmid=31992360|pmc=6986072}}</ref> |
The first company to bring a bioresorbable stent to market was [[Abbott Laboratories|Abbott Vascular]] which received European marketing approval in September 2012; the second was Elixir which received its CE mark in May 2013.<ref name="pmid26113479">{{cite journal | vauthors = Charpentier E, Barna A, Guillevin L, Juliard JM | title = Fully bioresorbable drug-eluting coronary scaffolds: A review | journal = Archives of Cardiovascular Diseases | volume = 108 | issue = 6–7 | pages = 385–397 | year = 2015 | pmid = 26113479 | doi = 10.1016/j.acvd.2015.03.009 | doi-access = }}</ref><ref name="www.fiercemedicaldevices.com">Damian Garde for Fierce Medical Devices. 22 May 2013 [http://www.fiercemedicaldevices.com/story/boston-scientific-elixir-make-waves-europcr-2013/2013-05-22 Boston Scientific, Elixir make waves at EuroPCR 2013] {{Webarchive|url=https://web.archive.org/web/20160116032410/http://www.fiercemedicaldevices.com/story/boston-scientific-elixir-make-waves-europcr-2013/2013-05-22 |date=16 January 2016 }}</ref><ref name="bioresorbable-2">{{cite journal|title=Bioresorbable vascular stents and drug-eluting stents in treatment of coronary heart disease: a meta-analysis|first1=Le|last1=Ni|first2=Hao|last2=Chen|first3=Zhurong|last3=Luo|first4=Yunqiang|last4=Yu|date=28 January 2020|journal=Journal of Cardiothoracic Surgery|volume=15|issue=1|pages=26|doi=10.1186/s13019-020-1041-5|doi-access=free |pmid=31992360|pmc=6986072}}</ref> |
||
| Line 165: | Line 165: | ||
Despite the initial promise, the first-generation bioresorbable stents faced some challenges. For instance, Absorb bioresorbable stent by Abbott was found to perform poorly when compared to current-generation drug-eluting stents, with several trials showing high rates of stent thrombosis, target-lesion myocardial infarction, and target vessel revascularization. In 2017, Abbott pulled its bioabsorbable stent, Absorb, from the European market after negative press regarding the device.<ref name="Husten-2017">{{cite web |vauthors=Husten L |date=6 April 2017 |title=Abbott Pulls Troubled Absorb Stent From European Market |url=http://www.cardiobrief.org/2017/04/06/abbott-pulls-troubled-absorb-stent-from-european-market/ |website=CardioBrief |access-date=2 November 2017 |archive-date=21 January 2021 |archive-url=https://web.archive.org/web/20210121055215/http://www.cardiobrief.org/2017/04/06/abbott-pulls-troubled-absorb-stent-from-european-market/ |url-status=live }}</ref> [[Boston Scientific]] also announced termination of its Renuvia bioresorbable coronary stent program as studies showed higher risk of serious adverse events.<ref name="Densford-2017">{{cite web |vauthors=Densford F |date=31 July 2017 |title=Boston Scientific to end Renuvia bioresorbable coronary stent program – MassDevice |url=http://www.massdevice.com/boston-scientific-end-renuvia-bioresorbable-coronary-stent-program/ |work=+MassDevice |access-date=2 November 2017 |archive-date=7 November 2017 |archive-url=https://web.archive.org/web/20171107031435/http://www.massdevice.com/boston-scientific-end-renuvia-bioresorbable-coronary-stent-program/ |url-status=live }}</ref> |
Despite the initial promise, the first-generation bioresorbable stents faced some challenges. For instance, Absorb bioresorbable stent by Abbott was found to perform poorly when compared to current-generation drug-eluting stents, with several trials showing high rates of stent thrombosis, target-lesion myocardial infarction, and target vessel revascularization. In 2017, Abbott pulled its bioabsorbable stent, Absorb, from the European market after negative press regarding the device.<ref name="Husten-2017">{{cite web |vauthors=Husten L |date=6 April 2017 |title=Abbott Pulls Troubled Absorb Stent From European Market |url=http://www.cardiobrief.org/2017/04/06/abbott-pulls-troubled-absorb-stent-from-european-market/ |website=CardioBrief |access-date=2 November 2017 |archive-date=21 January 2021 |archive-url=https://web.archive.org/web/20210121055215/http://www.cardiobrief.org/2017/04/06/abbott-pulls-troubled-absorb-stent-from-european-market/ |url-status=live }}</ref> [[Boston Scientific]] also announced termination of its Renuvia bioresorbable coronary stent program as studies showed higher risk of serious adverse events.<ref name="Densford-2017">{{cite web |vauthors=Densford F |date=31 July 2017 |title=Boston Scientific to end Renuvia bioresorbable coronary stent program – MassDevice |url=http://www.massdevice.com/boston-scientific-end-renuvia-bioresorbable-coronary-stent-program/ |work=+MassDevice |access-date=2 November 2017 |archive-date=7 November 2017 |archive-url=https://web.archive.org/web/20171107031435/http://www.massdevice.com/boston-scientific-end-renuvia-bioresorbable-coronary-stent-program/ |url-status=live }}</ref> |
||
The development of bioresorbable stents continues, with various manufacturers proposing new stents.<ref>https://www.dicardiology.com/article/abbott-restarts-bioresorbable-stent-clinical-trials-new-esprit-below-knee-scaffold</ref> However, until data from their clinical trials emerge, it remains unclear whether fully bioresorbable stents will play any significant role in coronary interventions.<ref>https://openheart.bmj.com/content/9/2/e002107</ref><ref>https://link.springer.com/chapter/10.1007/978-3-031-38743-2_14</ref><ref>https://link.springer.com/content/pdf/10.1007/s11883-019-0816-4.pdf</ref> |
The development of bioresorbable stents continues, with various manufacturers proposing new stents.<ref>{{cite web | url=https://www.dicardiology.com/article/abbott-restarts-bioresorbable-stent-clinical-trials-new-esprit-below-knee-scaffold | title=Abbott Restarts Bioresorbable Stent Clinical Trials with New Esprit Below-the-knee Scaffold | date=3 September 2020 }}</ref> However, until data from their clinical trials emerge, it remains unclear whether fully bioresorbable stents will play any significant role in coronary interventions.<ref>{{cite journal | url=https://openheart.bmj.com/content/9/2/e002107 | pmid=36288820 | date=2022 | title=Bioresorbable vascular scaffolds versus conventional drug-eluting stents across time: A meta-analysis of randomised controlled trials | journal=Open Heart | volume=9 | issue=2 | pages=e002107 | doi=10.1136/openhrt-2022-002107 | pmc=9615997 | vauthors = Jackson-Smith E, Zioupos S, Banerjee P }}</ref><ref>{{cite book | chapter-url=https://link.springer.com/chapter/10.1007/978-3-031-38743-2_14 | doi=10.1007/978-3-031-38743-2_14 | chapter=Fully Bioresorbable Vascular Stents | title=Current Trends in Biomedical Engineering | date=2023 | pages=255–268 | isbn=978-3-031-38742-5 | vauthors = Malmonge SM, Cliquet C }}</ref><ref>{{cite journal | url=https://link.springer.com/content/pdf/10.1007/s11883-019-0816-4.pdf | doi=10.1007/s11883-019-0816-4 | title=The Current Literature on Bioabsorbable Stents: A Review | date=2019 | journal=Current Atherosclerosis Reports | volume=21 | issue=12 | page=54 | pmid=31768641 | s2cid=208278983 | vauthors = Omar WA, Kumbhani DJ }}</ref> |
||
Due to challenges in developing resorbable stents, many manufacturers have focused efforts on targeting or reducing drug release through bioabsorbable-polymer coatings. Boston Scientific's Synergy bioabsorbable polymer stent has been shown potential to reduce the length of dual antiplatelet therapy post-implantation.<ref name="Kirtane-2018">{{cite journal | vauthors = Kirtane AJ, Mauri L, Stoler R, Feldman R, Neumann FJ, Boutis L, Tahirkheli N, Toelg R, Othman I, Stein B, Windecker S |date=22 September 2018 |title=TCT-841 Baseline characteristics and 3-month outcomes of the EVOLVE Short DAPT Trial: A prospective investigation of abbreviated antiplatelet therapy in high bleeding risk patients treated with a thin-strut bioabsorbable polymer-coated, everolimus-eluting coronary stent |journal=Journal of the American College of Cardiology |language=en |volume=72 |issue=13 Supplement |pages=B335–B336 |doi=10.1016/j.jacc.2018.08.2086 |issn=0735-1097 |doi-access=free}}</ref> [[MicroPort|MicroPort's]] Firehawk target eluting stent has been shown to be non-inferior to traditional drug-eluting stents while using one-third of the amount of equivalent drug.<ref name="pmid30190206">{{cite journal | vauthors = Lansky A, Wijns W, Xu B, Kelbæk H, van Royen N, Zheng M, Morel MA, Knaapen P, Slagboom T, Johnson TW, Vlachojannis G, Arkenbout KE, Holmvang L, Janssens L, Ochala A, Brugaletta S, Naber CK, Anderson R, Rittger H, Berti S, Barbato E, Toth GG, Maillard L, Valina C, Buszman P, Thiele H, Schächinger V, Baumbach A | title = Targeted therapy with a localised abluminal groove, low-dose sirolimus-eluting, biodegradable polymer coronary stent (TARGET All Comers): a multicentre, open-label, randomised non-inferiority trial | journal = Lancet | volume = 392 | issue = 10153 | pages = 1117–1126 | date = September 2018 | pmid = 30190206 | doi = 10.1016/S0140-6736(18)31649-0 | s2cid = 52169067}}</ref> |
Due to challenges in developing resorbable stents, many manufacturers have focused efforts on targeting or reducing drug release through bioabsorbable-polymer coatings. Boston Scientific's Synergy bioabsorbable polymer stent has been shown potential to reduce the length of dual antiplatelet therapy post-implantation.<ref name="Kirtane-2018">{{cite journal | vauthors = Kirtane AJ, Mauri L, Stoler R, Feldman R, Neumann FJ, Boutis L, Tahirkheli N, Toelg R, Othman I, Stein B, Windecker S |date=22 September 2018 |title=TCT-841 Baseline characteristics and 3-month outcomes of the EVOLVE Short DAPT Trial: A prospective investigation of abbreviated antiplatelet therapy in high bleeding risk patients treated with a thin-strut bioabsorbable polymer-coated, everolimus-eluting coronary stent |journal=Journal of the American College of Cardiology |language=en |volume=72 |issue=13 Supplement |pages=B335–B336 |doi=10.1016/j.jacc.2018.08.2086 |issn=0735-1097 |doi-access=free}}</ref> [[MicroPort|MicroPort's]] Firehawk target eluting stent has been shown to be non-inferior to traditional drug-eluting stents while using one-third of the amount of equivalent drug.<ref name="pmid30190206">{{cite journal | vauthors = Lansky A, Wijns W, Xu B, Kelbæk H, van Royen N, Zheng M, Morel MA, Knaapen P, Slagboom T, Johnson TW, Vlachojannis G, Arkenbout KE, Holmvang L, Janssens L, Ochala A, Brugaletta S, Naber CK, Anderson R, Rittger H, Berti S, Barbato E, Toth GG, Maillard L, Valina C, Buszman P, Thiele H, Schächinger V, Baumbach A | title = Targeted therapy with a localised abluminal groove, low-dose sirolimus-eluting, biodegradable polymer coronary stent (TARGET All Comers): a multicentre, open-label, randomised non-inferiority trial | journal = Lancet | volume = 392 | issue = 10153 | pages = 1117–1126 | date = September 2018 | pmid = 30190206 | doi = 10.1016/S0140-6736(18)31649-0 | s2cid = 52169067}}</ref> |
||
Revision as of 16:42, 11 March 2024
| Drug-eluting stent | |
|---|---|
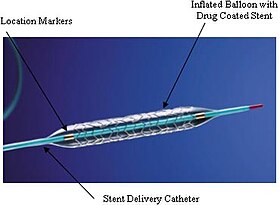 An example of a drug-eluting stent. This is the TAXUS Express2 Paclitaxel-Eluting Coronary Stent System, which releases paclitaxel. The system consists of a catheter delivery element, an inflation system, and the drug-eluting stent itself. They are marketed as one integrated system. | |
| ICD-9-CM | 00.55 |
| MeSH | D054855 |
A drug-eluting stent (DES) is a self-expanding tube made of a mesh-like material used to treat narrowed arteries (stenosis) in medical procedures. It is inserted into a narrowed artery using a balloon. Once the baloon inside the stent is inflated, the stent expands, pushing against the artery wall. The mesh design allows cells to grow through and around it, securing it in place.[1][2][3] The stent slowly releases a drug to prevent re-blockage of the artery. The release of the drug from the stant to prevent the growth of scar tissue and reduce the risk of stent restenosis, which is the narrowing of the stented area of an artery after treatment. A drug-eluting stent is different from other types of stents because it has a coating that delivers medication directly to the arterial wall. A DES is often made of metal alloys and can be inserted into blocked or narrowed arteries through a catheter placed in a peripheral artery, such as in the arm or leg. DES is fully integrated with a catheter delivery system and is viewed as one integrated medical device.[4][5][6]
DES medical devices are commonly used in the treatment of coronary artery disease (CAD), by opening narrowed arteries caused by atherosclerosis. Such stents are also commonly used to treat peripheral artery disease and at the site of other stenotic or occlusive lesions caused by a number of disease states Over the last three decades, coronary stenting has matured into a primary minimally invasive treatment tool in managing CAD.[7]
Coronary artery stenting is inherently tied to percutaneous coronary intervention (PCI) procedures. PCI is a minimally invasive procedure performed via a catheter (not by open-chest surgery), it is the medical procedure used to place a DES in narrowed coronary arteries. PCI procedures are performed by an interventional cardiologist using fluoroscopic imaging techniques to see the location of the required DES placement. PCI uses larger peripheral arteries in the arms or the legs to thread a catheter/DES device through the arterial system and place the DES in the narrowed coronary artery or arteries. Multiple stents are often used depending on the degree of blockage and the number of diseased coronary arteries that are being treated. As of 2023,[update] more than 90 percent of stents used in PCI procedures were DES.[8]

Drug-eluting stents in current use were approved by the FDA after clinical trials demonstrated they were superior to prior non-drug eluting or bare-metal stents.[9] There is significant clinical data relating to the use and outcomes of DES, general stents, PCI and coronary artery bypass graft (CABG) surgery.[10] As of 2023,[update] DES devices are a primary choice of interventional cardiologists and the use of bare metal stent (BMS) systems is less common.[11] DES products hold an advantage over BMS offerings. Based on long-term outcomes, the quality of life for individuals with cardiac conditions has been measurably enhanced through the use of DES technologies and PCI procedures.[12][13]
Uses

A drug-eluting stent is an implant that is used in angioplasty procedures to treat stenosis of blood vessels: the stent, which elutes drugs, is implanted into the blood vessel to help keep the vessel open and improve blood flow.[14][15][16] Specifically, drug-eluting stents are used in the treatment of various medical conditions usually at the site of stenotic or occlusive arterial lesions, but one of the primary medical uses is in the treatment of coronary artery disease.[17] Stents are inserted into narrowed coronary arteries, where the narrowing is primarily caused by atherosclerosis. Stents are then expanded to open up the narrowed artery. Such stents gradually release a drug compound that suppresses cellular growth, into the newly stented area, thereby reducing the potential for blockage within the stent area itself.[17] Such blockage is termed in-stent restenosis (ISR). This in-stent blockage is most often caused by excessive cell proliferation or thrombi (blood clots). Anticoagulation therapy (blood thinners), has become a standard treatment following the placement of DES. This therapy significantly reduces the occurrence of adverse events post-stenting.[18][8][19]
Percutaneous coronary intervention (PCI), a minimally invasive procedure, involves the placement of a drug-eluting stent (DES) in a coronary artery. This procedure, previously known as angioplasty with a stent, is considered non-surgical as it is performed through a small puncture in a peripheral artery, avoiding the need to open the chest wall. While bleeding from the puncture site was once a concern, advancements in PCI practices have mitigated this issue through the use of pressure bands and arterial closure systems. Modern DES/PCI procedures are generally painless, although some mild discomfort may be experienced.[20][21][22]
Clinical indications
| Coronary arteries providing blood to the heart. The blood vessels originate from the aorta and surround the heart. | |
|---|---|
 Showing the coronary arteries that are subject to narrowing - resulting in reduced blood supply to the cardiac muscle. | |
| Identifiers | |
| MeSH | D054855 |
| Anatomical terminology | |
PCI and stent placement are considered when someone shows signs of reduced blood flow in the arteries that supply the heart or when tests, such as different types of coronary artery imaging, show a blockage in those arteries.[23][24]
Symptoms can include:
- severe, pressure-like chest pain unrelieved by rest;
- shortness of breath, fatigue, lightheadedness;
- palpitations;
- atypical symptoms: nausea, vomiting, indigestion, confusion, back pain.[25]
In a medical setting, it's not very useful for doctors to rely solely on what people say about where their pain comes from or how it feels. This is because the way people describe chest pain caused by reduced blood flow to the heart can vary greatly and may not match what is typically taught in medical education or described in books and articles.[26][27]
Contraindications
DES is not recommended in some cases as it may do more harm than good. DES is not suitable:
- when individuals have a bleeding tendency;[28]
- when a coronary artery has no clear and identifiable narrowing;[29]
- when only one diseased coronary artery supplies oxygenated blood to the heart muscle. During stent placement, there is a short period of blood flow blockage by the balloon inflation. This blockage time is often longer than twenty seconds to allow the DES to expand and embed into the arterial wall. In this case, this time may be too long and cause serious events due to lack of blood to the heart muscle.[30]
Bleeding disorders make DES unsuitable because of the need for anticoagulation drugs (blood thinners) during the procedure and in post-stenting aftercare. Other factors that could rule out the use of stents include a history of in-stent blockage, bleeding problems, complex or unsuitable coronary anatomy, or a short life expectancy due to other serious medical conditions.[31]
Risks and complications
Risks from the procedure
Like all medical procedures, stent placement carries some risks. Risks include bleeding, allergic reactions to the contrast agents used to visualize the coronary arteries, and myocardial infarction. With percutaneous coronary intervention (PCI), the requirement for emergency coronary artery bypass graft (CABG) surgery has decreased as better practices have been introduced. In some situations, coronary stenting is permitted in hospitals without cardiac surgery facilities.[32] This remains controversial in the United States because of the rare but unpredictable risk of coronary artery perforation.[33]
Stent thrombosis risks
A complication of coronary stenting is stent thrombosis (blood clots). This occurs when a new clot forms within the stent and occludes blood flow, causing a heart attack.[34][35][36]
In-stent restenosis risks (ISR)
DES were designed to specifically combat issues of restenosis that occurred with older bare-metal stents (BMS). Though less frequent with drug-eluting stents, restenosis can still occur.[37]
Since the advent of DES technology, the incidence of ISR has significantly decreased.[38][39]
Usage outside the scope of typical regulatory approval
DES have been shown to be superior to BMS in reducing short-term complications of stenting in saphenous vein grafts.[40] However, the use of these stents in bypass grafts was not their originally intended use nor within the scope of originally regulatory approval (US FDA, European Medicines Agency, etc.). The practice of using a medical device or drug in a way not specified in the original or current approved labeling is often referred to as "off-label" use.[41]
In regions were cardiac stenting has become commonplace, think tanks and advocacy groups express concern about the overzealous use of stents,[42] because patients who received stents for unapproved reasons[43][44] often have worse outcomes compared to patients who received stents for approved uses.[45][46][47]
Clinical procedure
DES placement

People who receive a coronary stent have different needs depending on their medical condition. Some patients are actually having a heart attack and need immediate life-saving emergency care. Other patients are at high risk of having a heart attack in the very near future. For people from each of these groups, PCI procedures may vary slightly, with particular modifications as to how they are sedated, pain management, and broader intensive care issues such as breathing support.[48]
Many people who are not in critical care situations are usually fully awake during the PCI procedure and DES placement, but they receive local anesthetic at the site of catheter entry, to ensure there is no pain. Different sedation and pain management practices are used by different medical institutions and practitioners, but patient comfort is always a primary consideration.[49]
The catheter/stent system is inserted into the body by piercing a peripheral artery (an artery in the arm or leg) and moved through the arterial system to deliver the DES into the blocked coronary artery. The stent is then expanded to widen (open) blocked or narrowed coronary arteries (narrowed by plaque buildup), caused by a condition called atherosclerosis. Peripheral arterial access is usually through the femoral (upper leg) or the radial artery (arm/wrist) and less often done through the brachial or ulnar artery (wrist/arm).[50][51] In the past, controlling bleeding at the point of arterial access after the procedure was a problem. Modern arterial pressure bands and arterial closure systems now exist, which have helped control bleeding after the procedure, but it is still a concern.[52][53][54]
Modern catheter/stent systems are integrated medical devices, made of a guidewire, catheter, balloon, and stent. The stent tube mesh is initially collapsed onto the balloon of the device, and it is small enough to be passed through relatively narrow peripheral arteries. When in position, the balloon is inflated by introducing physiological saline, and this pushes the overlaying stent firmly into the diseased artery wall, inflation time and pressure are recorded during this placement procedure (consider an umbrella example, first closed and then opened). After placement, the balloon is deflated, and the device is removed from the body, leaving the expanded stent in place and opening up the artery.[55][56]
The interventional cardiologist decides how to treat the blockage in the best way during the PCI/DES placement, based on real-time data. The cardiologist uses imaging data provided by both intravascular ultrasound (IVUS), and fluoroscopic imaging (combined with a radiopaque dye). During the procedure, the information obtained from these two sources enables the cardiologist to track the path of the catheter-DES device as it moves through the arterial blood vessels. This information also helps determine both the location and characteristics of any plaque causing narrowing in the arteries. Data from these two techniques is used to correctly position the stent and to obtain detailed information relating to the coronary arterial anatomy. Given that this anatomy varies greatly among individuals, having this information becomes crucial for effective treatment. The obtained data is recorded on video and may be used in cases when further treatment is needed.[57][58][59]
Post-stenting recovery and rehabilitation
For many people the stenting procedure does not require staying in the hospital for any extended time period, most people leave the hospital the same day. Much of the time immediately after the stenting is spent in a recovery area to make sure the access site is not bleeding and to ensure vital signs are stable.[60]
In most hospital settings, the interventional cardiologist who performed the procedure will speak directly with the patient/family and give them information about how things went, and follow-up instructions. The nursing staff will keep an eye on the person's condition and use tools like ECG to monitor their heart. To prevent a blood clot from forming in the stent, medications are given right after the procedure. One common medication is plavix, which is a potent blood thinner that comes as a pill. Other medicines that thin the blood are also used, and it's typical to combine aspirin with plavix.[61] For people who have had a heart attack, the length of hospitalization is dependent on the degree of heart muscle damage caused by the event.[62]
A catheter with DES is a medical device, so people who receive it are given a medical device card. This card has information on the implanted DES and a medical device serial number. This information is important for future medical procedures, because it helps the doctors to know what type of device is in the person's body. Some arterial closure systems, which are devices that help to seal the access site after the procedure, are also medical devices and have their own informational cards.[63]
The access site is the place where the catheter enters the artery in the arm or leg. There is usually soreness and bruising at this site. This bruising and soreness usually get better after a week or so. People are advised to rest for a week or two and not to lift heavy things. This is mainly to make sure the access site heals well. It is normal to have follow-up appointments with a cardiologist or a primary care provider/general practitioner within a week or two of the procedure.[64][65]
People who get a coronary stent usually have more check-ups every three to six months for the first year, but this can vary. They usually do not need to have another coronary angiography, which is a test that uses a special dye and X-rays to see the arteries of the heart. If the doctors suspect that the heart disease is getting worse, they can prescribe a stress test, which is a test that measures how the heart works during physical activity. People who have symptoms or show signs of reduced blood flow to the heart in a stress test may need to have a diagnostic cardiac re-catheterization.[66]
After PCI-stenting procedures, physical examinations are important. People who have a high risk of complications or more complex coronary problems may need to have angiography. This may be the case even if the results of non-invasive stress tests, which are tests that measure how the heart works during physical activity, appear normal.[67]
Cardiac rehabilitation activities depend on many factors, but mainly on how much the heart muscle was damaged before the PCI/DES procedure. Many people who have this procedure have not had a heart attack, and their hearts may be fine. Others may have had a heart attack and their hearts may have trouble pumping oxygen-rich blood to the body. Rehabilitation activities are tailored to each person's needs.[68]
Efficacy
Benefits
DES are an improvement over older BMS devices as they reduce the chances of in-stent blockages. This reduces the incidence of serious post-stenting events such as, angina occurrence or recurrence, heart attacks, and death. They also reduce the likelihood of requiring another PCI procedure to open a blockage caused by the actual stent.[69]
The major benefit of drug-eluting stents (DES) when compared to bare-metal stents (BMS) is the prevention of in-stent restenosis (ISR). Restenosis is a gradual re-narrowing of the stented segment that occurs most commonly between 3–12 months after stent placement.[70] High rates of restenosis associated with BMS prompted the development of DES, which resulted in a reduction of ISR incidence to around 5-10%.[71] Continued development of newer generation DES have resulted in the near-elimination of BMS from clinical practice.[72]
Procedure outcomes
A key benefit of DES usage compared to BMS is a lower incidence of repeat revascularization procedures (re-stenting, invasive bypass surgeries etc.). Revascularization procedures are treatments that restore blood flow to parts of your heart that are not getting enough blood, a problem called ischemia. This can happen because of plaque buildup in the arteries of the heart, which can narrow or block them.[73] Rates of repeat revascularizations and stent thrombosis (blood clots) are significantly lower in those who received DES compared to BMS.[71]
Newer generations of DES devices have substantially improved safety outcomes, specifically regarding stent thrombosis, recurrent myocardial infarctions, and death.[73]
Design considerations
Summary
In designing a DES, the following factors are important issues:
- How the device functions as a structural scaffold keeping an artery open by physical means.
- Materials used for the stent fabrication, focusing on biocompatibility, extended mechanical performance in a biological environment such as human blood and located in an area that is in constant motion as the heart beats, and with some focus on the suitability for future patient imaging using MRI technologies, due to the high magnetic fields used in such imaging.[74]
- The drug-delivery features of the device.
- The drug that was chosen for the device, suitability in inhibiting restenosis, pharmacokinetics etc.
- DES products are integrated medical devices. Stents are part of a sophisticated PCI delivery system, and other components, such as the catheter design, are important considerations.
Newer stenting technologies that focus on the absorption of the stent over a period of time add further complexity to design considerations.[75][76][77]
Drugs commonly utilized in DES are everolimus, sirolimus, paclitaxel and biolimus. These drugs are also used for other purposes, that involve moderating the immune system or treating cancer. They work by inhibiting cell growth. In DES, they are used in very small amounts and for a short time, and only in the area where the stent is placed.[78]
Considerations for regulatory submission, assessment and approval

There are a number of very detailed medical device design considerations for DES products, these considerations are included in submissions for approval to regulatory authorities such as the US FDA:[77]
- Aspects of the design that relate to a DES as structural devices that keep an artery open by purely physical means.
- Choice of the construction materials, with a particular focus on biocompatibility, longevity in the human body, mechanical stress resistance and the suitability of the chosen material for future patient imaging using MRI technologies, due to the high magnetic fields used in such imaging.[74]
- Choice of a mechanism of the drug release: how long the drug lasts, and how to make the stent release the drug in a manner that inhibits in-stent restenosis.
- Choice of chemical agent the stent will deliver.
- Choice of the stent delivery technology as an integrated system: catheter design, placement visualization and assessment of the success of artery reperfusion (is the treated artery actually supplying cardiac muscle with sufficient oxygenated blood).
- Quality assurance considerations such as those defined in ISO 13485.
- Quality control considerations: what testing can be performed on each manufactured unit prior to release for sale to demonstrate its usage suitability.[75][76][79]
- Traceability issues, can a single stent be traced from the manufacturer to the patient it was implanted in. In the case of a recall of a product it is critical to be able to trace the stent from design, manufacture, and distribution to the patient.
The drug choice is a critical design element and determining its true effectiveness in inhibiting neointimal growth due to the proliferation of smooth muscle cells that would cause restenosis can be a design challenge. Much of the neointimal hyperplasia seems to be caused by inflammation.[79]
Vascular stents are classified by the US as class III medical devices,[80] meaning that they pose the highest risk to patients and are subject to both general and premarket approval, which requires clinical trials and scientific evidence of safety and effectiveness, as well as rigorous mechanical testing.[81] During the mechanical testing process, universal testing machines induce bending, stretching, twisting, and putting pressure on vascular stents from various angles.[80]
The specific properties of each type of stent and its intended use depend on the results of testing, and vice versa: different types of stents may need different or additional tests based on where they will be placed in the body and what they will be used for. Some of these additional tests might include checking how well the stent can withstand being crushed or bent out of shape, its resistance to getting kinks in it, whether it resists corrosion or damage over time, as well as making sure any coatings on the device remain intact.[80]
Alternatives to stenting procedures
Pharmacological therapy for coronary artery disease may be indicated instead of or in addition to invasive treatment. For those requiring percutaneous coronary intervention or surgery, medical therapy should be viewed as complementary to revascularization procedures, rather than an opposing strategy. Coronary artery bypass graft (CABG) surgery is an alternative to percutaneous coronary intervention (PCI) with drug-eluting stents (DES) for patients with ischemic left ventricular systolic dysfunction (LVSD). CABG is associated with lower risks of all-cause mortality, repeat revascularization, and myocardial infarction compared to PCI. However, there is no significant difference between the two procedures in terms of cardiovascular mortality, stroke, major adverse cardiovascular and cerebrovascular events, and ventricular tachycardia.[82]
History
The first procedure to treat blocked coronary arteries was coronary artery bypass graft surgery (CABG), wherein a section of vein or artery from elsewhere in the body is used to bypass the diseased segment of the coronary artery. In 1977, Andreas Grüntzig introduced percutaneous transluminal coronary angioplasty (PTCA), also called balloon angioplasty, in which a catheter was introduced through a peripheral artery and a balloon expanded to dilate the narrowed segment of the artery.[83]
As equipment and techniques improved, the use of PTCA rapidly increased, and by the mid-1980s, PTCA and CABG were being performed at equivalent rates.[84] Balloon angioplasty was generally effective and safe, but restenosis was frequent, occurring in about 30–40% of cases, usually within the first year after dilation. In about 3% of balloon angioplasty cases, failure of the dilation and acute or threatened closure of the coronary artery (often because of dissection) prompted emergency CABGs.[84]
Charles Theodore Dotter and Melvin Judkins had proposed using prosthetic devices inside arteries in the leg to maintain blood flow after dilation as early as 1964.[85] In 1986, Puel and Sigwart implanted the first coronary stent in a human patient.[86] Several trials in the 1990s showed the superiority of stent placement over balloon angioplasty. Restenosis was reduced because the stent acted as a scaffold to hold open the dilated segment of the artery. Acute closure of the coronary artery (and the requirement for emergency CABG) was reduced, because the stent repaired dissections of the arterial wall. By 1999, stents were used in 84% of percutaneous coronary interventions (i.e., those done via a catheter, and not by open-chest surgery).[86]
Early difficulties with coronary stents included a risk of early thrombosis (clotting) resulting in occlusion of the stent.[84] Coating stainless steel stents with other substances such as platinum or gold did not eliminate this problem.[86] High-pressure balloon expansion of the stent to ensure its full apposition to the arterial wall, combined with drug therapy using aspirin and another inhibitor of platelet aggregation (usually ticlopidine or clopidogrel) nearly eliminated this risk of early stent thrombosis.[84][86]
Though it occurred less frequently than with balloon angioplasty or other techniques, stents nonetheless remained vulnerable to restenosis, caused almost exclusively by neointimal tissue growth (tissue formation in the inner 'tube' structure of the artery). To address this issue, developers of drug-eluting stents used the devices themselves as a tool for delivering medication directly to the arterial wall. While initial efforts were unsuccessful, the release (elution) of drugs with certain specific physicochemical properties from the stent was shown in 2001 to achieve high concentrations of the drug locally, directly at the target lesion, with minimal systemic side effects.[87] As currently used in clinical practice, "drug-eluting" stents refers to metal stents that elute a drug designed to limit the growth of neointimal scar tissue, thus reducing the likelihood of stent restenosis.[88]
The first type of DES to be approved by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) were sirolimus-eluting stents (SES), which release a natural product called sirolimus,[89] an immunosuppressant drug.[90] SES were shown to reduce the need for repeat procedures and improve the outcomes of patients with coronary artery disease.[91][92][93] The sirolimus-eluting Cypher stent received CE mark approval in Europe in 2002, and then underwent a larger trial to demonstrate its safety and effectiveness for the US market.[94][95][96] The trial, published in 2003, enrolled 1058 patients with more complex lesions and confirmed the superiority of SES over bare metal stents in terms of angiographic and clinical outcomes.[97][98][99][100] Based on these results, the Cypher stent received FDA approval and was released in the US in 2003.[86] The FDA approval process for DES involves submitting an investigational device exemption (IDE) application to conduct clinical trials under 21 CFR Part 812, and then a premarket approval (PMA) application to obtain marketing authorization under 21 CFR Part 8144. The FDA assigns the primary review responsibility to the Center for Devices and Radiological Health (CDRH), but also consults with the Center for Drug Evaluation and Research (CDER) for the drug component of the combination product.
The second type of DES to be approved by the EMA and the FDA were paclitaxel-eluting stents (PES), which release another natural product called paclitaxel. PES also reduced the need for repeat procedures and improved the outcomes of patients with different types of lesions and risk factors. The paclitaxel-eluting Taxus stent received FDA approval and was launched in the US in 2004,[101] after a series of trials that compared it with a bare metal stent in various settings. The trials showed a significant reduction in target lesion revascularization and major adverse cardiac events with the Taxus stent at 9 and 12 months. Both SES and PES use natural products as the active agents to prevent the recurrence of blockages in the arteries.[102] These DES have changed the practice of interventional cardiology and have become the preferred treatment for many patients with coronary artery disease.[102][103][104]
The concept of using absorbable (also called biodegradable, bioabsorbable or bioresorbable)[105] materials in stents was first reported in 1878 by Huse who used magnesium wires as ligatures to halt the bleeding in vessels of three patients. Despite extensive search, the full name of this pioneer in the field remains elusive. [105][106] In 20th century, a resorbable stent tested in humans was developed by the Igaki Medical Planning Company in Japan and was constructed from poly-L-lactic acid (a form of polylactic acid); they published their initial results in 2000.[107] The German company Biotronik developed a magnesium absorbable (bioresorbable) stent and published clinical results in 2007.[107]
The first company to bring a bioresorbable stent to market was Abbott Vascular which received European marketing approval in September 2012; the second was Elixir which received its CE mark in May 2013.[108][109][110]
Despite the initial promise, the first-generation bioresorbable stents faced some challenges. For instance, Absorb bioresorbable stent by Abbott was found to perform poorly when compared to current-generation drug-eluting stents, with several trials showing high rates of stent thrombosis, target-lesion myocardial infarction, and target vessel revascularization. In 2017, Abbott pulled its bioabsorbable stent, Absorb, from the European market after negative press regarding the device.[111] Boston Scientific also announced termination of its Renuvia bioresorbable coronary stent program as studies showed higher risk of serious adverse events.[112]
The development of bioresorbable stents continues, with various manufacturers proposing new stents.[113] However, until data from their clinical trials emerge, it remains unclear whether fully bioresorbable stents will play any significant role in coronary interventions.[114][115][116]
Due to challenges in developing resorbable stents, many manufacturers have focused efforts on targeting or reducing drug release through bioabsorbable-polymer coatings. Boston Scientific's Synergy bioabsorbable polymer stent has been shown potential to reduce the length of dual antiplatelet therapy post-implantation.[117] MicroPort's Firehawk target eluting stent has been shown to be non-inferior to traditional drug-eluting stents while using one-third of the amount of equivalent drug.[118]
The first DES products available for treating patients were stainless steel alloys composed of iron, nickel, and chromium and were based on existing bare metal stents.[79] These stents were hard to visualize with medical imaging, posed a risk of causing allergic responses, and were difficult to deliver. Subsequent new alloys were used, namely cobalt-chrome and platinum chrome, with improved performance. Bioresorbable stents have been developed in which the stent itself dissolves over time.[16] Materials explored for use include magnesium, polylactic acid, polycarbonate polymers, and salicylic acid polymers.[107] Resorbable stents have held the promise of providing an acute treatment that would eventually allow the vessel to function normally, without leaving a permanent device behind.[119]
For the coating of DES, one to three or more layers of polymer can be used: a base layer for adhesion, a main layer that holds and elutes (releases) the drug into the arterial wall by contact transfer, and sometimes a top coat to slow down the release of the drug and extend its effect. The first few drug-eluting stents to be licensed used durable coatings. The first generation of coatings appears to have caused immunological reactions at times, and some possibly led to thrombosis. This has driven experimentation and the development of new coating approaches.[108]
Research directions
A research direction for a DES is to improve the material from which a device is made. The first-generation DES were made of stainless steel, while contemporary DES mainly consist of different kinds of alloys such as cobalt chromium and platinum chromium1. In the current generation DES, thinner struts are employed than in the first-generation DES with preserved radial strength and radio-opacity. The lower strut thickness is believed to be associated with better stent-related outcomes including target lesion revascularization, myocardial infarction, and stent thrombosis.[120]
Another area of research for DES focuses on polymers. The current generation DES includes both durable polymer-coated stents and biodegradable polymer-coated stents. It has been reported that the presence of a durable polymer in the body over a long period can lead to chronic inflammation and neoatherosclerosis. To address this potential limitation, researchers have developed biodegradable polymer DES as an alternative solution.[120][121][122]
Scientists are also studying different drugs that could be used in DES to prevent restenosis. These drugs, which have immunosuppressive[90] and anti-cancer properties, aim to inhibit the growth of smooth muscle cells. Additionally, there is a specific type of stent that features an extra layer of anti-CD4 antibodies on its struts. This additional layer is positioned on top of the polymer coating and aims to capture circulating endothelial progenitor cells. The goal behind this design is to promote improved healing of the blood vessel lining, known as the endothelium.[120][8]
A potential research focus for DES is the application of a polymer-free DES in clinical practice: moving away from polymer-based DES and instead using either a polymer-free DES or a drug-coated coronary stent. In the case of the polymer-free DES, it utilizes an abluminal coating of probucol to control the release of sirolimus. On the other hand, the drug-coated coronary stent has a micro-structured abluminal surface that allows for direct application of an anti-restenotic drug.[120][8]
Society and culture
Brand names and manufacturers
As of 2023[update] there are over 20 different types of drug-eluting stents available, with differences in features and characteristics.[123]
Economics
The economic evaluation of DES has been a topic of extensive research.[124] In 2007, the overall incremental cost-effectiveness ratio in Europe was €98,827 per quality-adjusted life-years gained. Avoiding one revascularization with DES would cost €4,794, when revascularization with BMS costs €3,2606.[125]
Controversies
There were controversies related to the use of DES. In 2012, a meta-analysis of clinical trial data[126] showed no benefit of the use of DES for people with stable coronary artery compared to treatment with drugs, yet, The New York Times interviewed David Brown, an author of the analysis, who said that more than half of patients with stable coronary artery disease were implanted with stents without even trying drug treatment and that he believed this happened because hospitals and doctors wanted to make more money.[127]
The interview sparked a debate among cardiologists, researchers, and patients about the appropriateness and effectiveness of DES for stable coronary artery disease: some agreed with the study's findings and questioned the overuse of stents,[128][129][130] while others criticized the study's methods and limitations and defended the benefits of stents, arguing that that the interviewee's statement was "outrageous and defamatory" and that he was "insulting the integrity of the entire profession.[131][132][133]
In 2013 the Times of India reported that DES were widely overused and that Indian distributors used profits from high markups on DES to bribe doctors to use them.[134][135]
In 2014 an investigation by the Maharashtra Food and Drug Administration found that high markups and bribery related to DES was still widespread.[136]
Intellectual property disputes
There have been several patent disputes related to drug-eluting stents. In one of them, Boston Scientific was found guilty of infringing upon a patent that was awarded to the University of Texas system in 2003 and ultimately licensed to TissueGen.[137][138][139] The patent concerns technology that TissueGen founder Kevin Nelson, Ph.D., developed while a faculty member at UT Arlington, designed to deliver drugs through an extruded fiber in an implanted vascular stent. Boston Scientific was therefore ordered to pay $42 million in lost royalties to TissueGen and the University of Texas.[137][138] The intellectual property case between Boston Scientific and TissueGen has finally reached a verdict after half a decade.[137]
Class action lawsuits
Drug-eluting stents have been associated with legal and ethical controversies, and there have been related class action lawsuits. In 2014, the former owners of St. Joseph Medical Center in Maryland settled a class action lawsuit for $37 million with hundreds of patients who received unnecessary DES implantation. The lawsuit alleged that Dr. Mark Midei, a cardiologist at the center, falsified the degree of coronary artery stenosis to justify the use of DES, exposing the patients to increased risks of thrombosis, bleeding, and infection. Another DES manufacturer, Cordis Corporation, a subsidiary of Johnson & Johnson, was involved in lawsuits from people who suffered adverse events from the Cypher Stent, a stainless-steel DES coated with sirolimus,[140][141] an immunosuppressant drug.[90] The Cypher Stent was approved by the FDA in 2003, but soon after, the FDA issued a Safety Warning following 290 reports of subacute thrombosis and at least 60 deaths related to the device.[140][141]
See also
- bioresorbable stent – medical stent that dissolves or is absorbed by the body;
- coronary stent – medical stent implanted into coronary arteries;
- drug-eluting implant – implant for delivering a drug.
References
- ^ "Stent: MedlinePlus Medical Encyclopedia".
- ^ "Drug-Eluting Stents Information".
- ^ Senst B, Goyal A, Basit H, Borger J (2024). "Drug Eluting Stent Compounds". StatPearls. PMID 30726034.
- ^ Stone G (2006). "Drug-eluting Stents - Current and Future Perspectives". Interventional Cardiology. 1 (1): 28–29. doi:10.15420/icr.2006.1.1.28. Archived from the original on 20 November 2023. Retrieved 20 November 2023.
- ^ "Onyx Frontier DES - Coronary Stents". Medtronic. Archived from the original on 19 November 2023. Retrieved 23 November 2023.
- ^ "Promus PREMIER™ Drug-Eluting Coronary Stent System". www.bostonscientific.com. Archived from the original on 23 November 2023. Retrieved 23 November 2023.
- ^ Iqbal J, Gunn J, Serruys PW (2013). "Coronary stents: historical development, current status and future directions". British Medical Bulletin. 106: 193–211. doi:10.1093/bmb/ldt009. PMID 23532779. S2CID 14423973.
- ^ a b c d Koźlik M, Harpula J, Chuchra PJ, Nowak M, Wojakowski W, Gąsior P (February 2023). "Drug-Eluting Stents: Technical and Clinical Progress". Biomimetics. 8 (1): 72. doi:10.3390/biomimetics8010072. PMC 9944483. PMID 36810403.
- ^ Anstrom KJ, Kong DF, Shaw LK, Califf RM, Kramer JM, Peterson ED, et al. (August 2008). "Long-term clinical outcomes following coronary stenting". Archives of Internal Medicine. 168 (15): 1647–1655. doi:10.1001/archinte.168.15.1647. PMID 18695078.
- ^ Spadaccio C, Benedetto U (July 2018). "Coronary artery bypass grafting (CABG) vs. percutaneous coronary intervention (PCI) in the treatment of multivessel coronary disease: quo vadis? -a review of the evidences on coronary artery disease". Annals of Cardiothoracic Surgery. 7 (4): 506–515. doi:10.21037/acs.2018.05.17. PMC 6082779. PMID 30094215.
- ^ Wu PN, Chen JH, Yang CP, Hsu JC (December 2022). "Advantages of DES over BMS in Preventing the Risk of Myocardial Infarction, Ischemic Stroke, and Mortality in Various Populations". Journal of Clinical Medicine. 12 (1): 24. doi:10.3390/jcm12010024. PMC 9820891. PMID 36614825.
- ^ Patel S, Patel KB, Patel Z, Konat A, Patel A, Doshi JS, et al. (March 2023). "Evolving Coronary Stent Technologies - A Glimpse Into the Future". Cureus. 15 (3): e35651. doi:10.7759/cureus.35651. PMC 10065169. PMID 37009355.
- ^ Habib A, Mori H, Yahagi K, Finn AV (3 February 2017). "Contemporary Drug-Eluting Stents and Vascular Response". EMJ Interventional Cardiology. 2 (1): 60–68. doi:10.33590/emj/10314324. ISSN 2053-423X. S2CID 13713278. Archived from the original on 5 November 2023. Retrieved 5 November 2023.
- ^ Majewska P, Oledzka E, Sobczak M (January 2020). "Overview of the latest developments in the field of drug-eluting stent technology". Biomater Sci. 8 (2): 544–551. doi:10.1039/c9bm00468h. PMID 31701961. S2CID 207966529.
- ^ Yasmin F, Jawed K, Moeed A, Ali SH (January 2024). "Efficacy of Intravascular Imaging-Guided Drug-Eluting Stent Implantation: A Systematic Review and Meta-Analysis of Randomized Clinical Trials". Curr Probl Cardiol. 49 (1 Pt A): 102002. doi:10.1016/j.cpcardiol.2023.102002. PMID 37544623. S2CID 260681557.
- ^ a b Waksman R (December 2017). "A new generation of drug-eluting stents: Indications and outcomes of bioresorbable vascular scaffolds". Cleveland Clinic Journal of Medicine. 84 (12 Suppl 4): e20–e24. doi:10.3949/ccjm.84.s4.05. PMID 29281608. S2CID 3663635.
- ^ a b Fischetti M (July 2006). "Vascular stents. Expanding use". Scientific American. 295 (1): 94–95. doi:10.1038/scientificamerican0706-94. PMID 16830686.
- ^ Cutlip DE (15 February 2017). "Dual Anti-platelet Therapy after Coronary Stenting: Rationale for Personalized Duration of Therapy". US Cardiol Rev. 11: 31–36. doi:10.15420/usc.2017:7:2. S2CID 108846490. Archived from the original on 20 November 2023. Retrieved 20 November 2023.
- ^ Slavin L, Chhabra A, Tobis JM (2007). "Drug-eluting stents: preventing restenosis". Cardiology in Review. 15 (1): 1–12. doi:10.1097/01.crd.0000200844.16899.fc. PMID 17172878. S2CID 24548420.
- ^ George S, Butler R, Nolan J, Mamas MA (30 June 2016). "Percutaneous Coronary Intervention and Bleeding Complications". EMJ Interventional Cardiology. 4 (1): 100–109. doi:10.33590/emjintcardiol/10314557. ISSN 2053-423X. S2CID 256147538. Archived from the original on 19 November 2023. Retrieved 19 November 2023.
- ^ Ahmad M, Mehta P, Reddivari AK, Mungee S (2023). "Percutaneous Coronary Intervention". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 32310583. Archived from the original on 4 June 2023. Retrieved 21 November 2023.
- ^ "Percutaneous Coronary Intervention (PCI)". Yale Medicine. Archived from the original on 22 October 2023. Retrieved 21 November 2023.
- ^ Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. (January 2022). "2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines". Circulation. 145 (3): e18–e114. doi:10.1161/CIR.0000000000001038. PMID 34882435. S2CID 245072028.
- ^ Arnold SV (2018). "Current Indications for Stenting: Symptoms or Survival CME". Methodist DeBakey Cardiovascular Journal. 14 (1): 7–13. doi:10.14797/mdcj-14-1-7. PMC 5880567. PMID 29623167.
- ^ Fred HL (2009). "Atypical chest pain: a typical humpty dumpty coinage". Texas Heart Institute Journal. 36 (5): 373–374. PMC 2763472. PMID 19876411.
- ^ Swap CJ, Nagurney JT (November 2005). "Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes". JAMA. 294 (20): 2623–2629. doi:10.1001/jama.294.20.2623. PMID 16304077.
- ^ "Coronary Artery Disease". U.S. Centers for Disease Control and Prevention (CDC). 19 July 2021. Archived from the original on 2 March 2015. Retrieved 24 November 2023.
- ^ Aroesty JM (19 June 2023). Cutlip D, Parikh N (eds.). "Patient education: Stenting for the heart (Beyond the Basics)". UpToDate. Archived from the original on 23 November 2023. Retrieved 23 November 2023.
- ^ Clark W, Burnes J (18 August 2016). "Angioplasty and Stent Insertion". InsideRadiology. Archived from the original on 29 November 2023. Retrieved 23 November 2023.
- ^ Iwamoto Y, Okamoto M, Hashimoto M, Fukuda Y, Iwamoto A, Iwasaki T, et al. (March 2012). "Better stent expansion by two-time inflation of stent balloon and its responsible mechanism". Journal of Cardiology. 59 (2): 160–166. doi:10.1016/j.jjcc.2011.12.003. PMID 22266460.
- ^ Griffin BP (2013). Manual of Cardiovascular Medicine (5th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 929–949. ISBN 978-1-4963-1260-0.
- ^ Peels JO, Hautvast RW, de Swart JB, Huybregts MA, Umans VA, Arnold AE, et al. (February 2009). "Percutaneous coronary intervention without on-site surgical back-up; two-years registry of a large Dutch community hospital". International Journal of Cardiology. 132 (1): 59–65. doi:10.1016/j.ijcard.2007.10.037. PMID 18241941.
- ^ "New Statement Shows PCI Without Surgery on Site Is as Safe as PCI With Surgery on Site". Society for Cardiovascular Angiography & Interventions (SCAI). Archived from the original on 23 November 2023. Retrieved 23 November 2023.
- ^ Kirtane AJ, Stone GW (September 2011). "How to minimize stent thrombosis". Circulation. 124 (11): 1283–1287. doi:10.1161/CIRCULATIONAHA.110.976829. PMID 21911796. S2CID 17063075.
- ^ Hassan S, Ali MN, Ghafoor B (April 2022). "Evolutionary perspective of drug eluting stents: from thick polymer to polymer free approach". Journal of Cardiothoracic Surgery. 17 (1): 65. doi:10.1186/s13019-022-01812-y. PMC 8981810. PMID 35379273.
- ^ Denktas AE, Grimes C (2018). "Percutaneous Coronary Intervention". In Levine GN (ed.). Cardiology Secrets. Elsevier. pp. 172–182. doi:10.1016/B978-0-323-47870-0.00019-2. ISBN 978-0-323-47870-0.
8 What is stent thrombosis? Stent thrombosis occurs when there is complete occlusion of the artery due to the formation of a thrombus in the stent. ... Stent thrombosis is a potentially catastrophic event and often presents as STEMI, requiring emergency revascularization. Stent thrombosis carries a mortality rate of 20% to 45%.
- ^ Serrano MC, Vavra AK, Jen M, Hogg ME, Murar J, Martinez J, et al. (May 2011). "Poly(diol-co-citrate)s as novel elastomeric perivascular wraps for the reduction of neointimal hyperplasia". Macromolecular Bioscience. 11 (5): 700–709. doi:10.1002/mabi.201000509. PMC 4068126. PMID 21341372.
- ^ Shlofmitz E, Ali ZA, Maehara A, Mintz GS, Shlofmitz R, Jeremias A (December 2020). "Intravascular Imaging-Guided Percutaneous Coronary Intervention: A Universal Approach for Optimization of Stent Implantation". Circulation. Cardiovascular Interventions. 13 (12): e008686. doi:10.1161/CIRCINTERVENTIONS.120.008686. PMID 33233934. S2CID 227169038.
- ^ Li M, Hou J, Gu X, Weng R, Zhong Z, Liu S (January 2022). "Incidence and risk factors of in-stent restenosis after percutaneous coronary intervention in patients from southern China". European Journal of Medical Research. 27 (1): 12. doi:10.1186/s40001-022-00640-z. PMC 8783476. PMID 35065663.
- ^ Lee MS, Shah AP, Aragon J, Jamali A, Dohad S, Kar S, et al. (December 2005). "Drug-eluting stenting is superior to bare metal stenting in saphenous vein grafts". Catheterization and Cardiovascular Interventions. 66 (4): 507–511. doi:10.1002/ccd.20498. PMID 16270361. S2CID 24315977.
- ^ Baldwin DE, Abbott JD, Trost JC, Vlachos HA, Selzer F, Glaser R, et al. (October 2010). "Comparison of drug-eluting and bare metal stents for saphenous vein graft lesions (from the National Heart, Lung, and Blood Institute Dynamic Registry)". The American Journal of Cardiology. 106 (7): 946–951. doi:10.1016/j.amjcard.2010.05.025. PMC 2945366. PMID 20854955.
- ^ Toleos A (31 October 2023). "PRESS RELEASE: Unnecessary coronary stents cost Medicare as much as $800 million per year". Lown Institute. Archived from the original on 3 November 2023. Retrieved 3 November 2023.
- ^ Win HK, Caldera AE, Maresh K, Lopez J, Rihal CS, Parikh MA, et al. (May 2007). "Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents". JAMA. 297 (18): 2001–2009. doi:10.1001/jama.297.18.2001. PMID 17488965.
- ^ Beohar N, Davidson CJ, Kip KE, Goodreau L, Vlachos HA, Meyers SN, et al. (May 2007). "Outcomes and complications associated with off-label and untested use of drug-eluting stents". JAMA. 297 (18): 1992–2000. doi:10.1001/jama.297.18.1992. PMID 17488964.
- ^ "Avoiding Overuse: Coronary Stents". Lown Institute Hospital Index. Archived from the original on 15 November 2023. Retrieved 21 November 2023.
- ^ Dixon SR, Grines CL, O'Neill WW (May 2010). "The year in interventional cardiology". Journal of the American College of Cardiology. 55 (20): 2272–86. doi:10.1016/j.jacc.2010.02.024. PMID 20466207.
- ^ Poorhosseini H, Kassaian SE, Aghajani H, Alidoosti M, Hajizeinali AM, Salarifar M, et al. (2012). "On-label and off-label use of drug-eluting stents: comparison of short- and long-term outcomes". Texas Heart Institute Journal. 39 (1): 24–29. PMC 3298939. PMID 22412223.
- ^ "Percutaneous coronary intervention". Heart and Stroke Foundation of Canada. Archived from the original on 23 November 2023. Retrieved 23 November 2023.
- ^ Song JW, Soh S, Shim JK (January 2020). "Monitored Anesthesia Care for Cardiovascular Interventions". Korean Circulation Journal. 50 (1): 1–11. doi:10.4070/kcj.2019.0269. PMC 6923237. PMID 31642214.
- ^ Stouffer III GA, Yadav PK, Todd JW, Yousuf MA (27 November 2019). Peter K (ed.). "Percutaneous Coronary Intervention (PCI) Technique: Access, Procedure, Anatomic and Physiologic Assessment". emedicine.medscape.com. Archived from the original on 13 November 2023. Retrieved 21 November 2023.
- ^ "TR BAND® Radial Compression Device". www.terumois.com. Archived from the original on 22 October 2023. Retrieved 21 October 2023.
- ^ Shuvy M, Ko DT (28 February 2014). "Bleeding after percutaneous coronary intervention: can we still ignore the obvious?". Open Heart. 1 (1): e000036. doi:10.1136/openhrt-2014-000036. PMC 4195920. PMID 25332793.
- ^ Thibert MJ, Fordyce CB, Cairns JA, Turgeon RD, Mackay M, Lee T, et al. (July 2021). "Access-Site vs Non-Access-Site Major Bleeding and In-Hospital Outcomes Among STEMI Patients Receiving Primary PCI". CJC Open. 3 (7): 864–871. doi:10.1016/j.cjco.2021.02.009. PMC 8347846. PMID 34401693.
- ^ Canfield J, Totary-Jain H (October 2018). "40 Years of Percutaneous Coronary Intervention: History and Future Directions". Journal of Personalized Medicine. 8 (4): 33. doi:10.3390/jpm8040033. PMC 6313463. PMID 30275411.
- ^ "Percutaneous Coronary Intervention (PCI)". Yale Medicine. Archived from the original on 22 October 2023. Retrieved 21 October 2023.
- ^ "Onyx Frontier DES - Coronary Stents". Medtronic. Archived from the original on 19 November 2023. Retrieved 19 November 2023.
- ^ "IVUS in PCI Guidance". American College of Cardiology. Archived from the original on 24 November 2023. Retrieved 21 November 2023.
- ^ Center for Devices and RadiologicalHealth (15 August 2023). "Fluoroscopy". FDA. Archived from the original on 11 November 2023. Retrieved 21 November 2023.
- ^ Goergen S (13 September 2016). "Iodine-containing contrast medium". InsideRadiology. Archived from the original on 7 July 2022. Retrieved 21 November 2023.
- ^ Dalal F, Dalal HM, Voukalis C, Gandhi MM (July 2017). "Management of patients after primary percutaneous coronary intervention for myocardial infarction". BMJ. 358: j3237. doi:10.1136/bmj.j3237. PMID 28729460. S2CID 46847680.
- ^ Radiological Society of North America (RSNA), American College of Radiology (ACR). "Angioplasty and Vascular Stenting". Radiologyinfo.org. Archived from the original on 21 November 2023. Retrieved 21 November 2023.
- ^ "Short Hospital Stays After Angioplasty Following Heart Attack Often Sufficient". American College of Cardiology. Archived from the original on 24 February 2024. Retrieved 21 November 2023.
- ^ "Coronary angioplasty and stents (PCI)". British Heart Foundation. Archived from the original on 21 November 2023. Retrieved 21 November 2023.
- ^ "Stents - What to Expect After Getting a Stent | NHLBI, NIH". www.nhlbi.nih.gov. 24 March 2022. Archived from the original on 21 November 2023. Retrieved 21 November 2023.
- ^ "Coronary angioplasty and stent insertion - Recovery". nhs.uk. 11 June 2018. Archived from the original on 24 December 2021. Retrieved 21 November 2023.
- ^ "Discharge advice after your coronary angiogram, angioplasty or stent insertion (PCI)". Hull University Teaching Hospitals NHS Trust. 9 April 2021. Archived from the original on 21 November 2023. Retrieved 21 November 2023.
- ^ "Stents - Living With a Stent | NHLBI, NIH". www.nhlbi.nih.gov. 24 March 2022. Archived from the original on 21 November 2023. Retrieved 21 November 2023.
- ^ "How Cardiac Rehabilitation Can Help Heal Your Heart". U.S. Centers for Disease Control and Prevention. 12 September 2022. Archived from the original on 27 November 2023. Retrieved 21 November 2023.
- ^ Piccolo R, Bonaa KH, Efthimiou O, Varenne O, Baldo A, Urban P, et al. (June 2019). "Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials". Lancet. 393 (10190): 2503–2510. doi:10.1016/S0140-6736(19)30474-X. PMID 31056295. S2CID 144207970.
- ^ Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R (November 2010). "In-stent restenosis in the drug-eluting stent era". Journal of the American College of Cardiology. 56 (23): 1897–1907. doi:10.1016/j.jacc.2010.07.028. PMID 21109112.
- ^ a b Bønaa KH, Mannsverk J, Wiseth R, Aaberge L, Myreng Y, Nygård O, et al. (September 2016). "Drug-Eluting or Bare-Metal Stents for Coronary Artery Disease". The New England Journal of Medicine. 375 (13): 1242–1252. doi:10.1056/NEJMoa1607991. PMID 27572953.
- ^ Shlofmitz E, Iantorno M, Waksman R (August 2019). "Restenosis of Drug-Eluting Stents: A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review". Circulation. Cardiovascular Interventions. 12 (8): e007023. doi:10.1161/CIRCINTERVENTIONS.118.007023. PMID 31345066. S2CID 198912657.
- ^ a b Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, et al. (June 2015). "Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis". Journal of the American College of Cardiology. 65 (23): 2496–2507. doi:10.1016/j.jacc.2015.04.017. hdl:11392/2415452. PMID 26065988.
- ^ a b Hanawa T (1 June 2009). "Materials for metallic stents". Journal of Artificial Organs. 12 (2): 73–79. doi:10.1007/s10047-008-0456-x. PMID 19536623. S2CID 20660118.
- ^ a b Gonzalo N, Macaya C (2012). "Absorbable stent: focus on clinical applications and benefits". Vascular Health and Risk Management. 8: 125–132. doi:10.2147/VHRM.S22551. PMC 3295634. PMID 22399857.
- ^ a b Pradhan A, Vishwakarma P, Vankar S, Sethi R (2019). ""The Unpredictable ABSORB" - Very Late Stent Thrombosis of Bioresorbable Vascular Scaffold". Heart Views. 20 (2): 65–69. doi:10.4103/HEARTVIEWS.HEARTVIEWS_18_19. PMC 6686611. PMID 31462962.
- ^ a b Lee DH, de la Torre Hernandez JM (August 2018). "The Newest Generation of Drug-eluting Stents and Beyond". European Cardiology. 13 (1): 54–59. doi:10.15420/ecr.2018:8:2. PMC 6159420. PMID 30310472. Archived from the original on 22 October 2023. Retrieved 21 October 2023.
- ^ Cassagnol M, Maha S. "Drug-Eluting Stents". www.uspharmacist.com. Archived from the original on 21 November 2023. Retrieved 21 November 2023.
- ^ a b c Nikam N, Steinberg TB, Steinberg DH (2014). "Advances in stent technologies and their effect on clinical efficacy and safety". Medical Devices: Evidence and Research. 7: 165–178. doi:10.2147/MDER.S31869. PMC 4051714. PMID 24940085. S2CID 5022642.
- ^ a b c Yalcin D (29 November 2019). "Mechanical Testing of Vascular Stents". ADMET. Archived from the original on 23 November 2023. Retrieved 23 November 2023.
- ^ "Overview of Medical Device Classification and Reclassification". FDA. 3 November 2018. Archived from the original on 19 November 2023. Retrieved 19 November 2023.
- ^ Jaiswal V, Ang SP, Shrestha AB, Joshi A, Ishak A, Chia JE, et al. (June 2023). "Percutaneous coronary intervention versus coronary artery bypass grafting among patients with left ventricular systolic dysfunction: a systematic review and meta-analysis". Annals of Medicine and Surgery. 85 (6): 2849–2857. doi:10.1097/MS9.0000000000000634. PMC 10289746. PMID 37363575.
- ^ Grüntzig AR, Senning A, Siegenthaler WE (July 1979). "Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty". The New England Journal of Medicine. 301 (2): 61–68. doi:10.1056/NEJM197907123010201. PMID 449946.
- ^ a b c d Baim DS (2005) [1958]. "Percutaneous Coronary Revascularization". In Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL (eds.). Harrison's Principles of Internal Medicine (16th ed.). New York: McGraw-Hill. pp. 1459–1462.
- ^ Dotter CT, Judkins MP (November 1964). "Transluminal Treatment of Arteriosclerotic Obstruction. Description of a New Technic and a Preliminary Report of Its Application". Circulation. 30 (5): 654–670. doi:10.1161/01.CIR.30.5.654. PMID 14226164.
- ^ a b c d e Serruys PW, Kutryk MJ, Ong AT (February 2006). "Coronary-artery stents". The New England Journal of Medicine. 354 (5): 483–495. doi:10.1056/NEJMra051091. PMID 16452560. S2CID 13647055.
- ^ Hwang CW, Wu D, Edelman ER (July 2001). "Physiological transport forces govern drug distribution for stent-based delivery". Circulation. 104 (5): 600–605. doi:10.1161/hc3101.092214. PMID 11479260.
- ^ Ellis SG, Holme DR (2006). Strategic Approaches in Coronary Intervention. Lippincott Williams & Wilkins. ISBN 978-0-7817-4294-8. Archived from the original on 20 October 2023. Retrieved 13 May 2015.
- ^ "Guideline on the development of medicinal substances contained in drug-eluting (medicinal substance-eluting) coronary stents" (PDF). London: European Medicines Agency. 22 March 2007. Archived (PDF) from the original on 8 February 2024. Retrieved 8 February 2024.
- ^ a b c Mukherjee T, Shah BV (October 2005). "Sirolimus: a new immunosuppressant". J Assoc Physicians India. 53: 885–90. PMID 16459533.
- ^ "Sirolimus-Eluting Stents for Chronic Total Coronary Occlusions - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. 5 March 2007. Archived from the original on 13 April 2021. Retrieved 8 February 2024.
- ^ "Sirolimus-eluting Stents With Biodegradable Polymer Versus an Everolimus-eluting Stents - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. 16 October 2018. Archived from the original on 15 May 2021. Retrieved 8 February 2024.
- ^ "Clinical Trial on the Efficacy and Safety of Sirolimus-Eluting Stent (MiStent® System) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. 3 September 2020. Archived from the original on 7 April 2021. Retrieved 8 February 2024.
- ^ Mauri L, Hsieh Wh, Massaro JM, Ho KK, D'Agostino R, Cutlip DE (8 March 2007). "Stent Thrombosis in Randomized Clinical Trials of Drug-Eluting Stents". New England Journal of Medicine. 356 (10): 1020–1029. doi:10.1056/NEJMoa067731. PMID 17296821. Archived from the original on 3 May 2023. Retrieved 8 February 2024 – via CrossRef.
- ^ "A Randomized, Double-blind Study to Assess Paclitaxel-eluting Stents in Treatment of Longer Lesions - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Archived from the original on 6 April 2021. Retrieved 8 February 2024.
- ^ Dawkins KD, Grube E, Guagliumi G, Banning AP, Zmudka K, Colombo A, et al. (22 November 2005). "Clinical Efficacy of Polymer-Based Paclitaxel-Eluting Stents in the Treatment of Complex, Long Coronary Artery Lesions From a Multicenter, Randomized Trial: Support for the Use of Drug-Eluting Stents in Contemporary Clinical Practice". Circulation. 112 (21): 3306–3313. doi:10.1161/CIRCULATIONAHA.105.552190. PMID 16286586. Archived from the original on 8 February 2024. Retrieved 8 February 2024 – via CrossRef.
- ^ "Clinical Trial Results - Eluvia Drug-Eluting Stent". www.bostonscientific.com. Archived from the original on 8 February 2024. Retrieved 8 February 2024.
- ^ Galløe AM, Thuesen L, Kelbæk H, Thayssen P, Rasmussen K, Hansen PR, et al. (30 January 2008). "Comparison of Paclitaxel- and Sirolimus-Eluting Stents in Everyday Clinical PracticeThe SORT OUT II Randomized Trial". JAMA. 299 (4): 409–416. doi:10.1001/jama.299.4.409. PMID 18230778. Archived from the original on 24 February 2024. Retrieved 8 February 2024 – via Silverchair.
- ^ Dai Y, Wang R, Chen F, Zhang Y, Liu Y, Huang H, et al. (12 November 2021). "Clinical outcomes in 2481 unselected real-world patients treated with a polymer-free sirolimus-eluting stent: 3 years results from the NANO multicenter Registry". BMC Cardiovascular Disorders. 21 (1): 537. doi:10.1186/s12872-021-02356-0. PMC 8588634. PMID 34772347.
- ^ Saito S, Bennett J, Nef HM, Webster M, Namiki A, Takahashi A, et al. (November 2023). "First randomised controlled trial comparing the sirolimus-eluting bioadaptor with the zotarolimus-eluting drug-eluting stent in patients with de novo coronary artery lesions: 12-month clinical and imaging data from the multi-centre, international, BIODAPTOR-RCT" (PDF). eClinicalMedicine. 65. doi:10.1016/j.eclinm.2023.102304. PMC 10725075. PMID 38106564.
- ^ Commissioner Oo (3 November 2018). "Jurisdictional Update: Drug-Eluting Cardiovascular Stents". FDA. Archived from the original on 8 December 2023. Retrieved 8 February 2024 – via www.fda.gov.
- ^ a b Uhrin P, Wang D, Mocan A, Waltenberger B, Breuss JM, Tewari D, et al. (November 2018). "Vascular smooth muscle cell proliferation as a therapeutic target. Part 2: Natural products inhibiting proliferation". Biotechnology Advances. 36 (6): 1608–1621. doi:10.1016/j.biotechadv.2018.04.002. PMID 29678389. S2CID 5027489.
- ^ "Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials - The Lancet" (PDF). Archived (PDF) from the original on 8 February 2024. Retrieved 8 February 2024.
- ^ Li M, Tu H, Yan Y, Guo Z, Zhu H, Niu J, et al. (21 September 2023). "Meta-analysis of outcomes from drug-eluting stent implantation in femoropopliteal arteries". PLOS ONE. 18 (9): e0291466. Bibcode:2023PLoSO..1891466L. doi:10.1371/journal.pone.0291466. PMC 10513203. PMID 37733656.
- ^ a b Aljihmani L, Alic L, Boudjemline Y, Hijazi ZM, Mansoor B, Serpedin E, et al. (2019). "Magnesium-Based Bioresorbable Stent Materials: Review of Reviews". Journal of Bio- and Tribo-Corrosion. 5. doi:10.1007/s40735-019-0216-x. S2CID 80811999.
- ^ Witte F, Eliezer A (2012). "Biodegradable Metals". Degradation of Implant Materials. pp. 93–109. doi:10.1007/978-1-4614-3942-4_5. ISBN 978-1-4614-3941-7.
- ^ a b c Ormiston JA, Serruys PW (June 2009). "Bioabsorbable coronary stents". Circulation. Cardiovascular Interventions. 2 (3): 255–260. doi:10.1161/CIRCINTERVENTIONS.109.859173. PMID 20031723.
- ^ a b Charpentier E, Barna A, Guillevin L, Juliard JM (2015). "Fully bioresorbable drug-eluting coronary scaffolds: A review". Archives of Cardiovascular Diseases. 108 (6–7): 385–397. doi:10.1016/j.acvd.2015.03.009. PMID 26113479.
- ^ Damian Garde for Fierce Medical Devices. 22 May 2013 Boston Scientific, Elixir make waves at EuroPCR 2013 Archived 16 January 2016 at the Wayback Machine
- ^ Ni L, Chen H, Luo Z, Yu Y (28 January 2020). "Bioresorbable vascular stents and drug-eluting stents in treatment of coronary heart disease: a meta-analysis". Journal of Cardiothoracic Surgery. 15 (1): 26. doi:10.1186/s13019-020-1041-5. PMC 6986072. PMID 31992360.
- ^ Husten L (6 April 2017). "Abbott Pulls Troubled Absorb Stent From European Market". CardioBrief. Archived from the original on 21 January 2021. Retrieved 2 November 2017.
- ^ Densford F (31 July 2017). "Boston Scientific to end Renuvia bioresorbable coronary stent program – MassDevice". +MassDevice. Archived from the original on 7 November 2017. Retrieved 2 November 2017.
- ^ "Abbott Restarts Bioresorbable Stent Clinical Trials with New Esprit Below-the-knee Scaffold". 3 September 2020.
- ^ Jackson-Smith E, Zioupos S, Banerjee P (2022). "Bioresorbable vascular scaffolds versus conventional drug-eluting stents across time: A meta-analysis of randomised controlled trials". Open Heart. 9 (2): e002107. doi:10.1136/openhrt-2022-002107. PMC 9615997. PMID 36288820.
- ^ Malmonge SM, Cliquet C (2023). "Fully Bioresorbable Vascular Stents". Current Trends in Biomedical Engineering. pp. 255–268. doi:10.1007/978-3-031-38743-2_14. ISBN 978-3-031-38742-5.
- ^ Omar WA, Kumbhani DJ (2019). "The Current Literature on Bioabsorbable Stents: A Review" (PDF). Current Atherosclerosis Reports. 21 (12): 54. doi:10.1007/s11883-019-0816-4. PMID 31768641. S2CID 208278983.
- ^ Kirtane AJ, Mauri L, Stoler R, Feldman R, Neumann FJ, Boutis L, et al. (22 September 2018). "TCT-841 Baseline characteristics and 3-month outcomes of the EVOLVE Short DAPT Trial: A prospective investigation of abbreviated antiplatelet therapy in high bleeding risk patients treated with a thin-strut bioabsorbable polymer-coated, everolimus-eluting coronary stent". Journal of the American College of Cardiology. 72 (13 Supplement): B335–B336. doi:10.1016/j.jacc.2018.08.2086. ISSN 0735-1097.
- ^ Lansky A, Wijns W, Xu B, Kelbæk H, van Royen N, Zheng M, et al. (September 2018). "Targeted therapy with a localised abluminal groove, low-dose sirolimus-eluting, biodegradable polymer coronary stent (TARGET All Comers): a multicentre, open-label, randomised non-inferiority trial". Lancet. 392 (10153): 1117–1126. doi:10.1016/S0140-6736(18)31649-0. PMID 30190206. S2CID 52169067.
- ^ Gogas BD, Farooq V, Onuma Y, Serruys PW (2012). "The ABSORB bioresorbable vascular scaffold: an evolution or revolution in interventional cardiology?" (PDF). Hellenic Journal of Cardiology. 53 (4): 301–309. PMID 22796817. Archived (PDF) from the original on 9 August 2017. Retrieved 13 January 2016.
- ^ a b c d Saito Y, Kobayashi Y (September 2023). "Contemporary coronary drug-eluting and coated stents: an updated mini-review (2023)". Cardiovascular Intervention and Therapeutics. 39 (1): 15–17. doi:10.1007/s12928-023-00954-7. PMID 37656338. S2CID 261430959.
- ^ "Drug-Eluting Stents Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023-2028". Research and Markets Ltd. Archived from the original on 24 November 2023. Retrieved 24 November 2023.
- ^ "Drug-eluting Stent Market Size, share & Growth Research Report". www.bccresearch.com. Archived from the original on 24 November 2023. Retrieved 24 November 2023.
- ^ Nicolas J, Pivato CA, Chiarito M, Beerkens F, Cao D, Mehran R (May 2023). "Evolution of drug-eluting coronary stents: a back-and-forth journey from the bench to bedside". Cardiovascular Research. 119 (3): 631–646. doi:10.1093/cvr/cvac105. PMID 35788828.
- ^ Willich SN, Müller-Riemenschneider F, McBride D, Silber S, Kuck KH, Nienaber CA, et al. (February 2012). "Health economic evaluation of the use of drug-eluting stents: First results from the Drug-Eluting Stent Registry (DES.de)". Herz. 38 (1): 57–64. doi:10.1007/s00059-012-3581-5. PMID 22301731. S2CID 25967413.
- ^ Kuukasjärvi P, Räsänen P, Malmivaara A, Aronen P, Sintonen H (2007). "Economic evaluation of drug-eluting stents: a systematic literature review and model-based cost-utility analysis". International Journal of Technology Assessment in Health Care. 23 (4): 473–479. doi:10.1017/S0266462307070560. PMID 17937836. S2CID 31846667.
- ^ Stergiopoulos K, Brown DL (February 2012). "Initial coronary stent implantation with medical therapy vs medical therapy alone for stable coronary artery disease: meta-analysis of randomized controlled trials". Archives of Internal Medicine. 172 (4): 312–319. doi:10.1001/archinternmed.2011.1484. PMID 22371919.
- ^ Bakalar N (27 February 2012). "No Extra Benefits Are Seen in Stents for Coronary Artery Disease". The New York Times. ISSN 0362-4331. Archived from the original on 24 November 2023. Retrieved 24 November 2023.
- ^ Kolata G (16 November 2019). "Surgery for Blocked Arteries Is Often Unwarranted, Researchers Find". The New York Times. ISSN 0362-4331. Archived from the original on 29 December 2022. Retrieved 24 November 2023.
- ^ Guzick DS (2020). An introduction to the US health care industry: balancing care, cost, and access. Baltimore: Johns Hopkins University Press. ISBN 978-1-4214-3882-5.
- ^ Vani A (6 November 2013). "To Stent or Not to Stent?". Clinical Correlations. New York University (NYU) Department of Medicine. Archived from the original on 24 November 2023. Retrieved 24 November 2023.
- ^ Kureshi F, Jones PG, Buchanan DM, Abdallah MS, Spertus JA (September 2014). "Variation in patients' perceptions of elective percutaneous coronary intervention in stable coronary artery disease: cross sectional study". BMJ. 349: g5309. doi:10.1136/bmj.g5309. PMC 4157615. PMID 25200209.
- ^ "Expert Answers on E.M.D.R." Consults Blog. The New York Times. 16 March 2012. Archived from the original on 21 January 2024. Retrieved 24 November 2023.
- ^ "Patients Before Profits (1 Letter)". The New York Times. 5 March 2012. ISSN 0362-4331. Archived from the original on 24 November 2023. Retrieved 24 November 2023.
- ^ John DA (30 January 2013). "Unnecessary stent usage worries doctors across India". Times of India. Archived from the original on 26 June 2017. Retrieved 13 May 2015.
- ^ Hollmer M (30 January 2013). "In India, a call to halt financial incentives for stent use". Fierce Medical Devices. Archived from the original on 18 May 2015. Retrieved 13 May 2015.
- ^ Nagarajan R (15 September 2014). "Profits from medical devices used to bribe doctors?". The Times of India. Archived from the original on 16 December 2016. Retrieved 13 May 2015.
- ^ a b c Park A (3 February 2023). "Boston Scientific fined $42M in stent patent lawsuit". Fierce Biotech. Archived from the original on 24 November 2023. Retrieved 24 November 2023.
- ^ a b "Boston Scientific hit with $42M verdict in drug-eluting stents IP case". 2 February 2023. Archived from the original on 8 February 2024. Retrieved 8 February 2024.
- ^ "Annual Review of Medical Device Patent Litigation | Publications | Insights". Faegre Drinker Biddle & Reath LLP. Archived from the original on 24 November 2023. Retrieved 24 November 2023.
- ^ a b "Heart Stent Problems - Drug Eluting Stent Lawsuits". Saiontz & Kirk, P.A. Archived from the original on 24 November 2023. Retrieved 24 November 2023.
- ^ a b "Heart Stent Class Action Lawsuit". The Schmidt Firm, PLLC. Archived from the original on 24 November 2023. Retrieved 24 November 2023.
