5-Oxo-eicosatetraenoic acid: Difference between revisions
→5-Oxo-ETE's target cells: correcting reference |
→5-Oxo-ETE's target cells: adding other 5-oxo-ETE target cells |
||
| Line 68: | Line 68: | ||
== 5-Oxo-ETE's target cells == |
== 5-Oxo-ETE's target cells == |
||
=== Inflammatory cells === |
|||
5-Oxo-ETE is a potent in vitro stimulator and/or enhancer of [[chemotaxis]] (i.e. directional migration) and, depending on the cell type, various other responses such as [[degranulation]] (i.e. release of granule-bound enzymes), oxidative metabolism (i.e. generation of [[reactive oxygen species]]), and production of mediators such as various arachidonic acid metabolites and [[platelet-activating factor]] in human eosinophils, [[basophil]]s, neutrophils, and [[monocyte]]s.<ref>J Allergy Clin Immunol. 2005 Nov;116(5):1014-9.</ref><ref>Int Immunol. 2006 Nov;18(11):1575-83. Epub 2006 Sep 19</ref><ref>J Immunol. 1996 Nov 15;157(10):4664-71</ref><ref>J Immunol. 1996 Jul 1;157(1):336-42</ref><ref>J Biol Chem. 2004 Jul 2;279(27):28159-64</ref><ref>Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Review</ref> Indeed, the injection of 5-oxo-ETE into the skin of humans causes the local accumulation of eosinophils.<ref>J Allergy Clin Immunol. 2003 Oct;112(4):768-74</ref> The activity of 5-oxo-ETE on the two cell types known to be involved in allergy, eosinophils and basophils, suggests that it may be involved in promoting allergic reactions possibly by attracting through chemotaxis these cells to nascent sites of allergy and/or through stimulating these cells to release granule-bound enzymes, reactive oxygen species, or other promoters of allergic reactions. |
5-Oxo-ETE is a potent in vitro stimulator and/or enhancer of [[chemotaxis]] (i.e. directional migration) and, depending on the cell type, various other responses such as [[degranulation]] (i.e. release of granule-bound enzymes), oxidative metabolism (i.e. generation of [[reactive oxygen species]]), and production of mediators such as various arachidonic acid metabolites and [[platelet-activating factor]] in human eosinophils, [[basophil]]s, neutrophils, and [[monocyte]]s.<ref>J Allergy Clin Immunol. 2005 Nov;116(5):1014-9.</ref><ref>Int Immunol. 2006 Nov;18(11):1575-83. Epub 2006 Sep 19</ref><ref>J Immunol. 1996 Nov 15;157(10):4664-71</ref><ref>J Immunol. 1996 Jul 1;157(1):336-42</ref><ref>J Biol Chem. 2004 Jul 2;279(27):28159-64</ref><ref>Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Review</ref> Indeed, the injection of 5-oxo-ETE into the skin of humans causes the local accumulation of eosinophils and to lesser extents neutrophils and monocyte-derived macrophages.<ref>J Allergy Clin Immunol. 2003 Oct;112(4):768-74</ref> The activity of 5-oxo-ETE on the two cell types known to be involved in allergy-based inflammation, eosinophils and basophils, suggests that it may be involved in promoting allergic reactions possibly by attracting through chemotaxis these cells to nascent sites of allergy and/or through stimulating these cells to release granule-bound enzymes, reactive oxygen species, or other promoters of allergic reactions. 5-Oxo-ETE contracts smooth muscle and organ-cultured bronchi isolated from guinea pigs,<ref>Am J Physiol Lung Cell Mol Physiol. 2004 Oct;287(4):L631-40</ref><ref>Prostaglandins Other Lipid Mediat. 2007 Jan;82(1-4):30-41</ref> but relaxes bronchi isolated from human lung; this suggests that 5-oxo-ETE is not directly involved in the [[bronchoconstriction]]) that occurs in eosinophil-based allergic [[asthma]] reactions in humans. 5-Oxo-ETE's activity on human cells involved in non-allergic inflammatory diseases viz., neutrophils and monocytes, as well as its ability to attack these cell types to the skin of humans suggest that 5-oxo-ETE may also be involved in the broad category of non-allergic inflammatory diseases including those involving host defense against pathogens. |
||
=== Cancer cells === |
|||
5-Oxo-ETE (or other 5-HETE family member) stimulates the growth and/or survival of human cell lines derived from cancers of the prostate;<ref name="Can. Res. 62:6817-6819, 2002"/><ref>{{cite journal | vauthors = Ghosh J, Myers CE | title = Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 95 | issue = 22 | pages = 13182–13187 | date = Oct 1998 | pmid = 9789062 | pmc = 23752 | doi = 10.1073/pnas.95.22.13182 | bibcode = 1998PNAS...9513182G }}</ref><ref>{{cite journal | vauthors = Rodríguez-Blanco G, Burgers PC, Dekker LJ, Ijzermans JJ, Wildhagen MF, Schenk-Braat EA, Bangma CH, Jenster G, Luider TM | title = Serum levels of arachidonic acid metabolites change during prostate cancer progression | journal = The Prostate | volume = 74 | issue = 6 | pages = 618–627 | date = May 2014 | pmid = 24435810 | doi = 10.1002/pros.22779 }}</ref> breast;<ref name="Biochim. Biophys 2005"/><ref>{{cite journal | vauthors = Grant GE, Rubino S, Gravel S, Wang X, Patel P, Rokach J, Powell WS | title = Enhanced formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by cancer cells in response to oxidative stress, docosahexaenoic acid and neutrophil-derived 5-hydroxy-6,8,11,14-eicosatetraenoic acid | journal = Carcinogenesis | volume = 32 | issue = 6 | pages = 822–828 | date = Jun 2011 | pmid = 21393477 | pmc = 3146358 | doi = 10.1093/carcin/bgr044 }}</ref> lung;<ref>{{cite journal | vauthors = Avis IM, Jett M, Boyle T, Vos MD, Moody T, Treston AM, Martínez A, Mulshine JL | title = Growth control of lung cancer by interruption of 5-lipoxygenase-mediated growth factor signaling | journal = The Journal of Clinical Investigation | volume = 97 | issue = 3 | pages = 806–813 | date = Feb 1996 | pmid = 8609238 | pmc = 507119 | doi = 10.1172/JCI118480 }}</ref><ref>{{cite journal |title=none| journal = Biomed. Chromatogr. | volume = 8 | pages = 817–821 | date = 2009 }}</ref> Ovary;<ref name="Biochim. Biophys 2005"/><ref>clin. Can. Res. 13:5736-5744, 200, Epub ahead of print</ref> colon;,<ref>{{cite journal | vauthors = Hussey HJ, Tisdale MJ | title = Inhibition of tumour growth by lipoxygenase inhibitors | journal = British Journal of Cancer | volume = 74 | issue = 5 | pages = 683–687 | date = Sep 1996 | pmid = 8795568 | pmc = 2074717 | doi=10.1038/bjc.1996.422}}</ref> and pancreas.<ref name="Oncology 65:285-294, 2003"/><ref>{{cite journal | vauthors = Ding XZ, Iversen P, Cluck MW, Knezetic JA, Adrian TE | title = Lipoxygenase inhibitors abolish proliferation of human pancreatic cancer cells | journal = Biochemical and Biophysical Research Communications | volume = 261 | issue = 1 | pages = 218–213 | date = Jul 1999 | pmid = 10405349 | doi = 10.1006/bbrc.1999.1012 }}</ref> These preclinical studies suggest that 5-oxo-ETE (or other 5-HETE family member) may contribute to the cited cancers progression in humans. |
|||
=== Steroidogenic cells === |
|||
5-oxo-ETE stimulates human [[H295R]] adrenocortical cells to increase transcription of steroidogenic acute regulatory protein messenger RNA and produce [[aldosterone]] and [[progesterone]] by an apparent OXER1-dependent pathway.<ref>Mol. Cell. Endocrin. 371:71-78, 2013</ref> |
|||
=== Other cell types === |
|||
5-Oxo-ETE induces an isotonic volume reduction in guinea pig intestinal crypt epithelial cells.<ref>J Pharmacol Exp Ther. 1999 Nov;291(2):511-6</ref> |
|||
== 5-Oxo-ETE's interaction with other stimuli == |
== 5-Oxo-ETE's interaction with other stimuli == |
||
Revision as of 16:03, 4 November 2015
| |
| Names | |
|---|---|
| IUPAC name
5-oxo-6E,8Z,11Z,14Z-eicosatetraenoate
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H30O3 | |
| Molar mass | 318.45 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
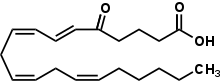
5-Oxo-eicosatetraenoic acid (5-oxo-6E,8Z,11Z,14Z-eicosatetraenoate or 5-oxo-ETE) is the most potent naturally occurring member of the 5-HETE family of arachidonic acid metabolites that stimulate a variety of cell types including most particularly the human eosinophil by binding to the OXER1 receptor.[1] Preclinical studies suggest that 5-oxo-ETE may be an important mediator of human allergy as well as certain other inflammatory and non-inflammatory pathophysiological responses.[2][3][4]
5-Oxo-ETE production
Cells make 5-oxo-ETE by a) oxygenating arachidonic acid with arachidonate 5-lipoxygenase (ALOX5) to form 5(S)-hydroperoxy-eicosatetraenoic acid (5(S)-HpETE); b) reducing 5(S)-HpETE with cellular peroxidases to form 5(S)-hydroxy-eicosatetraeonic acid (5(S)-HETE), and (c) oxidizing 5(S)-HETE with microsome-bound nicotinamide adenine dinucleotide phosphate (NADP+)-dependent dehydrogenase (5-hydroxyicosanoid dehydrogenase [5-HEDH]) to form 5-oxo-ETE (see 5-HETE).[5] (5-HEDH has little or no such effect on the R stereoisomer of 5(S)-HETE viz., 5(R)-HETE.) 5-Oxo-ETE can also be made form either 5(S)-HpETE (and possibly 5(R)-HpEPE) by the action of cytochrome P450 (CYP) enzymes such as CYP1A1, CYP1A2, CYP1B1, and CYP2S1.;[6] from 5(S)-HETE (and probably 5(R)-HETE) by the non-enzymatic attack with heme or various other dehydrating agents;[7] and from the conversion of 5-(S)-HpETE and 5(R)-HpETE to 5-oxo-ETE by the action of a mouse macrophage 50-60 kilodalton cytosolic protein.[8] The contribution of the latter three pathways to the physiological production of 5-oxo-ETE has not been fully studied; most attention has focused on the 5-HEDH pathway.
5-HEDH acts reversibly with the direction of its 5-(S)-HETE and 5-oxo-ETE interconversions determined by ambient NADPH/NADP+ ratios[9][10] Cells containing high levels of NADPH compared to NADP+ make little or no 5-oxo-ETE from endogenous 5-HETE and rapidly convert exogenous 5-oxo-ETE to 5-(S)-HETE whereas cells containing low levels of NADPH compared to NADP+ convert sizable portions of 5-(S)-HETE to 5-oxo-ETE.[11] Since most cells normally maintain high NADPH/NADP+ ratios, they make little or no 5-oxo-ETE from 5(S)-HETE and rapidly convert exogenous 5-oxo-ETE to 5-(S)-HETE.[12][13] However, cells undergoing aging, senescence, apoptosis, oxidative stress, or other conditions that raise their levels of reactive oxygen species (e.g. superoxide anion, oxygen radicals, and peroxides) either physiologically (e.g. human phagocytes engulfing bacteria) or pathologically (e.g. oxidatively challenged B-lymphocytes) use up NADP+, have low NADPH/NADP+ ratios, and therefore readily convert 5(S)-HETE to 5-oxo-ETE.[14][15][16][17][18][19] Thus, many pathological conditions, including those involving oxidative stress such as occurs in rapidly growing cancers, may be important promoters of 5-oxo-ETE accumulation in vivo.
Human neutrophils, monocytes, eosinophils, B-lymphocytes, dendritic cell, platelets, airway epithelial cells and smooth muscle cells, vascular endothelial cells, and skin keratinocytes have been found and/or suggested to make 5-oxo-ETE from endogenous or exogenous 5-HETE, particularly under conditions of oxidative stress; cell lines derived from human cancers such as those from breast, prostate, lung, colon, and various types of leukemia have likewise been shown to be producers of 5-oxo-ETE.[20] The production of 5-oxo-ETE by these cells often involves transcellular metabolism wherein cells of one type make 5(S)-HETE and release it to nearby cells of a second type which then oxidize the 5(S)-HETE to 5-oxo-ETE. This sharing of responsibilities, at least in in vitro studies, typically involves the limited number of cell types that express active 5-lipoxygenase and therefore act as donors that deliver 5(S)-HETE to the far larger number of cell types that contain 5-HEDH and/or possess lower NADPH/NADP+ ratios than the donor cells. The transcellular production of 5-oxo-eicosatetraenoates has been demonstrated in vitro with human neutrophils as the 5(S)-HETE producers and human PC-3 prostate cancer cells, platelets, and monocyte-derived dendritic cells as the oxidizing cells.[21][22] It is theorized that this transcellular metabolism occurs in vivo and provides a mechanism for controlling 5-oxo-ETE production by allowing it to occur of be greatly augmented at sites were 5-lipoxygenase-containg cells congregate with cell types possessing 5-HEDH and favorable NADPH/NADP+ ratios; such sites might include those involving allergy, inflammation, oxidative stress, and rapidly growing cancers.
5-Oxo-ETE metabolism
As indicated in the previous section, 5-oxo-ETE is readily converted to 5(S)-HETE by 5-HEDH in cells containing high NADPH/NADP+ ratios. Human neutrophils, an important model cell for investigating 5-oxo-ETE production, take up 5-oxo-ETE and reduce it to 5(S)-HETE; they also form appreciable amounts of 5(S),20-dihydroxy-ETE and far smaller amounts of 5-oxo,20-hydroxy-ETE probably by the action of the ω-hydroxylase cytochrome P450 enzyme, CYP453A.[23][24] The cells also incorporate the 5(S)-HETE product of 5-oxo-ETE but little or no 5-oxo-ETE itself as an ester into their various phospholipid and glycerolipid pools; however, isolated neutrophil plasma membranes, which lack appreciable 5-HEDH activity, do esterify 5-oxo-ETE into these lipid pools.[25]
In addition to the above metabolic pathways: a) human eosinophils may use Arachidonate 15-lipoxygenase-1 (or possibly Arachidonate 15-lipoxygenase-2 to oxygenate 5-oxo-ETE to make 5-oxo-15-(S)-hydroperoxy-ETE which is then converted to 5-oxo-15(S)-hydroxy-ETE; b) human platelets use 12-lipoxygenase to oxygenate 5-oxo-ETE to make 5-oxo-12(S)-hydroxy-eicosatetraenoat which is then converted to 5-oxo-12(S)-hydroxy-eicosatetraenoate (5-oxo-12-hydroxy-ETE);[26] c) a cytochrome P450 enzyme in mouse macrophages converts 5-oxo-ETE to 5-oxo-18-hydroxy-ETE (5-oxo-18-HETE) which is either attacked by a 5-keto-reductase (possibly 5-HEDH) to form 5,18-dihydroxy-eicosatetraenoic acid (5,18-diHETE) or by a Δ6-reductase to form 5-oxo-18-hydroxy-eicosatrienoic acid (5-oxo-18-HETrE) which is then reduced by a 5-keto-reductase (possibly 5-HEDH) to 5,18-dihydroxy-eicosatetrienoic acid (5,18-diHETrE); d) a cytochrome P450 enzyme in mouse macrophages converts 5-oxo-ETE to 5-oxo-19-hydroxy-eicosatetraenoic acid (5-oxo-19-HETE) which is then either reduced by a keto reductase (possibly 5-HEDH) to 5,19-dihydroxy-eicosatetraenoic acid (5,19-diHETE) or by a Δ6 reductase to 5-oxo-19-hydroxy-eicosatrienoic acid (5-oxo-19-HETrE);[27] and e) leukotriene C4 synthase in mice metabolizes 5-oxo-ETE to 5-oxo-7-glutathionyl-8,11,14-eicosatrienoic acid (FOG7).[28][29]
5-Oxo-ETE's mechanism of action
Studies in human neutrophils first detected a plasma membrane-localized site which reversibly bound 5-oxo-ETE;[30] this binding site had the attributes of a Gi alpha subunit-linked G protein-coupled receptor based on the ability of 5-oxo-ETE to activate this class of membrane G proteins by a pertussis toxin-sensitive mechanism.[31] The binding and G protein-activating potencies of various members of the 5-HETE family of agonists including 5-oxo-ETE and 5-oxo-15-hydroxy-ETE paralleled their ability to stimulate neutrophil functional responses (see 5-HETE). Subsequently, this receptor was cloned by several groups who termed it oxoeicosanoid receptor 1 (OXER1), OXE, OXE-R, hGPCR48, HGPCR48, and R527 (its gene is termed OXE1 or OXER1), and, as predicted by the cited binding studies, found it coupled with and activated the G protein complex composed of the Gi alpha subunit (Gαi) and G beta-gamma complex (Gβγ).[32][33][34][35][26][36][37] When bound by 5-oxo-ETE or other 5-HETE family member OXER1 triggered this G protein complex to dissociate into its Gαi and Gβγ components.[38][39] with Gβγ being responsible for activating many of the signal pathways that lead to the cellular functional responses elicited by the 5-HETE family of agonists.[40] The cell-activation pathways stimulated by OXER1 include those evoking rises in cytosolic calcium ion levels,[41][42][43] as well as those activating MAPK/ERK, p38 mitogen-activated protein kinases, cytosolic Phospholipase A2, PI3K/Akt, protein kinase C beta (PKCβ), and protein kinase C epsilon (PKCε).[35][44][45][46][47][48] 5-Oxo-ETE also stimulates human neutrophils to activate cytosolic phospholipase A2 (cPLA2) and increase these cells' activation of cPLA2 as well as cPLA2-induced release of arachidonic acid elicited by other stimuli.[49]
OXER1 mRNA is highly expressed in human blood eosinophils, neutrophils, spleen, lung, liver and kidney; this mRNA is expressed at lower levels in human basophils, monocytes, lung macrophages, various cancer cell lines, and an adrenocortical cell line.[50] Orthologs of OXER1 are found in various mammalian species including cats and opossums as well as several species of fish; however, mice and rats lack a clear ortholog of OXER1.[51][52]
5-Oxo-ETE and 5-oxo-15(S)-hydroxy-ETE but not 5-hydroxy members of the 5-HETE family such as 5-(S)-HETE also activate peroxisome proliferator-activated receptor gamma (PPARγ). This activation does not proceed through OXER1; rather, it involves the direct binding of the oxo analog to PPARγ with 5-oxo-15-(S)-hydroxy-ETE being more potent than 5-oxo-ETE in binding and activating PPARγ.[53] The Activation of OXER1 receptor and PPARγ by the oxo analogs can have opposing effects on cell function. For example, 5-oxo-ETE-bound OXER1 stimulates whereas 5-oxo-ETE-bound PPARγ inhibits the proliferation of various types of human cancer cell lines; this results in 5-oxo-ETE and 5-oxo-15-(S)-HETE having considerably less potency than anticipated in stimulating these cancer cells to proliferate relative to the potency of 5-(S)-HETE, a relationship not closely following the potencies of these three compounds in activating OXER1.[53]
5-Oxo-ETE's target cells
Inflammatory cells
5-Oxo-ETE is a potent in vitro stimulator and/or enhancer of chemotaxis (i.e. directional migration) and, depending on the cell type, various other responses such as degranulation (i.e. release of granule-bound enzymes), oxidative metabolism (i.e. generation of reactive oxygen species), and production of mediators such as various arachidonic acid metabolites and platelet-activating factor in human eosinophils, basophils, neutrophils, and monocytes.[54][55][56][57][58][59] Indeed, the injection of 5-oxo-ETE into the skin of humans causes the local accumulation of eosinophils and to lesser extents neutrophils and monocyte-derived macrophages.[60] The activity of 5-oxo-ETE on the two cell types known to be involved in allergy-based inflammation, eosinophils and basophils, suggests that it may be involved in promoting allergic reactions possibly by attracting through chemotaxis these cells to nascent sites of allergy and/or through stimulating these cells to release granule-bound enzymes, reactive oxygen species, or other promoters of allergic reactions. 5-Oxo-ETE contracts smooth muscle and organ-cultured bronchi isolated from guinea pigs,[61][62] but relaxes bronchi isolated from human lung; this suggests that 5-oxo-ETE is not directly involved in the bronchoconstriction) that occurs in eosinophil-based allergic asthma reactions in humans. 5-Oxo-ETE's activity on human cells involved in non-allergic inflammatory diseases viz., neutrophils and monocytes, as well as its ability to attack these cell types to the skin of humans suggest that 5-oxo-ETE may also be involved in the broad category of non-allergic inflammatory diseases including those involving host defense against pathogens.
Cancer cells
5-Oxo-ETE (or other 5-HETE family member) stimulates the growth and/or survival of human cell lines derived from cancers of the prostate;[44][63][64] breast;[53][65] lung;[66][67] Ovary;[53][68] colon;,[69] and pancreas.[45][70] These preclinical studies suggest that 5-oxo-ETE (or other 5-HETE family member) may contribute to the cited cancers progression in humans.
Steroidogenic cells
5-oxo-ETE stimulates human H295R adrenocortical cells to increase transcription of steroidogenic acute regulatory protein messenger RNA and produce aldosterone and progesterone by an apparent OXER1-dependent pathway.[71]
Other cell types
5-Oxo-ETE induces an isotonic volume reduction in guinea pig intestinal crypt epithelial cells.[72]
5-Oxo-ETE's interaction with other stimuli
5-Oxo-ETE and platelet-activating factor exhibit synergism in activating human neutrophils and eosinophils; that is, the combined agents elicit responses at relatively small doses that not only far exceed the responses elicited by either agent alone as well as the level of responses expected by adding the responses elicited by each agent.
References
- ^ Biochim Biophys Acta. 2015 Apr;1851(4):340-55. doi: 10.1016/j.bbalip.2014.10.008
- ^ Biochim Biophys Acta. 2015 Apr;1851(4):340-55. doi: 10.1016/j.bbalip.2014.10.008. Epub 2014 Oct 29. Review.
- ^ Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001
- ^ Biochem Pharmacol. 2015 Aug 1;96(3):247-55. doi: 10.1016/j.bcp.2015.05.009
- ^ Powell WS, Gravelle F, Gravel S (Sep 1992). "Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes". The Journal of Biological Chemistry. 267 (27): 19233–19241. PMID 1326548.
- ^ Bui P, Imaizumi S, Beedanagari SR, Reddy ST, Hankinson O (Feb 2011). "Human CYP2S1 metabolizes cyclooxygenase- and lipoxygenase-derived eicosanoids". Drug Metabolism and Disposition. 39 (2): 180–90. doi:10.1124/dmd.110.035121. PMID 21068195.
- ^ Powell WS, Rokach J (Apr 2015). "Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid". Biochimica Et Biophysica Acta. 1851 (4): 340–55. doi:10.1016/j.bbalip.2014.10.008. PMID 25449650.
- ^ Zarini S, Murphy RC (Mar 2003). "Biosynthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid from 5-hydroperoxyeicosatetraenoic acid in the murine macrophage". The Journal of Biological Chemistry. 278 (13): 11190–6. doi:10.1074/jbc.M208496200. PMID 12547823.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Biochem J. 2007 Apr 1;403(1):157-65
- ^ Free Radic Biol Med. 2011 May 15;50(10):1297-304. doi:10.1016/j.freeradbiomed.2011.02.010
- ^ Biochim Biophys Acta. 2014 Oct 29. pii: S1388-1981(14)00217-0. doi: 10.1016/j.bbalip.2014.10.008. [Epub ahead of print] Review
- ^ Biol Chem. 1992 Sep 25;267(27):19233-41>
- ^ J Biol Chem. 1998 Dec 4;273(49):32535-41
- ^ J Biol Chem. 2004 Sep 24;279(39):40376-84
- ^ Free Radic Biol Med. 2007 Mar 1;42(5):654-64
- ^ Free Radic Biol Med. 2009 Jul 1;47(1):62-71. doi:10.1016/j.freeradbiomed
- ^ Free Radic Biol Med. 2011 May 15;50(10):1297-304. doi: 0.1016/j.freeradbiomed.2011.02.010
- ^ Biosci Rep. 2014 May 23;34(3). pii: e00108. doi: 10.1042/BSR20130136
- ^ Biochim Biophys Acta. 2015 Apr;1851(4):340-55. doi: 10.1016/j.bbalip.2014.10.008. Epub 2014 Oct 29. Review
- ^ Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001.
- ^ Inflamm Res. 2000 Nov;49(11):633-8
- ^ Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Review
- ^ J Biol Chem. 1998 Dec 4;273(49):32535-41
- ^ Prog Lipid Res. 2013 Oct;52(4):651-65. doi:10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Review>
- ^ J Biol Chem. 1998 Dec 4;273(49):32535-41
- ^ a b Powell WS, Rokach J (Oct 2013). "The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor". Progress in Lipid Research. 52 (4): 651–665. doi:10.1016/j.plipres.2013.09.001. PMID 24056189.
- ^ Hevko JM, Bowers RC, Murphy RC (Feb 2001). "Synthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid and identification of novel omega-oxidized metabolites in the mouse macrophage". The Journal of Pharmacology and Experimental Therapeutics. 296 (2): 293–305. PMID 11160610.
- ^ Bowers RC, Hevko J, Henson PM, Murphy RC (Sep 2000). "A novel glutathione containing eicosanoid (FOG7) chemotactic for human granulocytes". The Journal of Biological Chemistry. 275 (39): 29931–4. doi:10.1074/jbc.C000502200. PMID 10924496.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hevko JM, Murphy RC (Mar 2002). "Formation of murine macrophage-derived 5-oxo-7-glutathionyl-8,11,14-eicosatrienoic acid (FOG7) is catalyzed by leukotriene C4 synthase". The Journal of Biological Chemistry. 277 (9): 7037–43. doi:10.1074/jbc.M108942200. PMID 11748223.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ J Biol Chem. 1998 Dec 4;273(49):32535-41
- ^ J Immunol. 2000 Mar 15;164(6):3345-52.
- ^ J Biol Chem. 1998 Dec 4;273(49):32535-41
- ^ Hosoi T, Koguchi Y, Sugikawa E, Chikada A, Ogawa K, Tsuda N, Suto N, Tsunoda S, Taniguchi T, Ohnuki T (Aug 2002). "Identification of a novel human eicosanoid receptor coupled to G(i/o)". The Journal of Biological Chemistry. 277 (35): 31459–31465. doi:10.1074/jbc.M203194200. PMID 12065583.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Jones CE, Holden S, Tenaillon L, Bhatia U, Seuwen K, Tranter P, Turner J, Kettle R, Bouhelal R, Charlton S, Nirmala NR, Jarai G, Finan P (Mar 2003). "Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils". Molecular Pharmacology. 63 (3): 471–477. doi:10.1124/mol.63.3.471. PMID 12606753.
- ^ a b Hosoi T, Sugikawa E, Chikada A, Koguchi Y, Ohnuki T (Sep 2005). "TG1019/OXE, a Galpha(i/o)-protein-coupled receptor, mediates 5-oxo-eicosatetraenoic acid-induced chemotaxis". Biochemical and Biophysical Research Communications. 334 (4): 987–995. doi:10.1016/j.bbrc.2005.06.191. PMID 16039985.
- ^ Koike D, Obinata H, Yamamoto A, Takeda S, Komori H, Nara F, Izumi T, Haga T (Mar 2006). "5-Oxo-eicosatetraenoic acid-induced chemotaxis: identification of a responsible receptor hGPCR48 and negative regulation by G protein G(12/13)". Journal of Biochemistry. 139 (3): 543–549. doi:10.1093/jb/mvj060. PMID 16567419.
- ^ Pharmacol. Rev. 56:149-157,2004
- ^ J Biol Chem. 1998 Dec 4;273(49):32535-41
- ^ Hosoi T, Koguchi Y, Sugikawa E, Chikada A, Ogawa K, Tsuda N, Suto N, Tsunoda S, Taniguchi T, Ohnuki T (Aug 2002). "Identification of a novel human eicosanoid receptor coupled to G(i/o)". The Journal of Biological Chemistry. 277 (35): 31459–31465. doi:10.1074/jbc.M203194200. PMID 12065583.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ J. Immunol. 192: 4474–4782. 2014.
{{cite journal}}: CS1 maint: untitled periodical (link) - ^ Biochem Biophys Res Commun. 1987 Oct 29;148(2):575-81
- ^ FEBS Lett. 1988 Nov 21;240(1-2):163-6
- ^ Oflaherty, J.T.; Cordes, J.; Redman, J.; Thomas, M.J. (1993). "5-Oxo-Eicosatetraenoate, a Potent Human Neutrophil Stimulus". Bioche. Biophys. Res. Commun. 192 (1): 129–134. doi:10.1006/bbrc.1993.1391.
- ^ a b Can. Res. 62: 6817–6819. 2002.
{{cite journal}}: CS1 maint: untitled periodical (link) - ^ a b Ding XZ, Tong WG, Adrian TE (2003). "Multiple signal pathways are involved in the mitogenic effect of 5(S)-HETE in human pancreatic cancer". Oncology. 65 (4): 285–294. doi:10.1159/000074640. PMID 14707447.
- ^ Wijkander J, O'Flaherty JT, Nixon AB, Wykle RL (Nov 1995). "5-Lipoxygenase products modulate the activity of the 85-kDa phospholipase A2 in human neutrophils". The Journal of Biological Chemistry. 270 (44): 26543–26549. doi:10.1074/jbc.270.44.26543. PMID 7592874.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sarveswaran S, Thamilselvan V, Brodie C, Ghosh J (Dec 2011). "Inhibition of 5-lipoxygenase triggers apoptosis in prostate cancer cells via down-regulation of protein kinase C-epsilon". Biochimica Et Biophysica Acta. 1813 (12): 2108–2117. doi:10.1016/j.bbamcr.2011.07.015. PMC 3541030. PMID 21824498.
- ^ Sarveswaran S, Ghosh J (Aug 2013). "OXER1, a G protein-coupled oxoeicosatetraenoid receptor, mediates the survival-promoting effects of arachidonate 5-lipoxygenase in prostate cancer cells". Cancer Letters. 336 (1): 185–195. doi:10.1016/j.canlet.2013.04.027. PMC 3892773. PMID 23643940.
- ^ J Biol Chem. 1995 Nov 3;270(44):26543-9
- ^ Biochim Biophys Acta. 2015 Apr;1851(4):340-55. doi:10.1016/j.bbalip.2014.10.008. Epub 2014 Oct 29>
- ^ Powell WS, Rokach J (2013). "The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor". Prog. Lipid Res. 52 (4): 651–65. doi:10.1016/j.plipres.2013.09.001. PMID 24056189.
- ^ Biochem Pharmacol. 2015 Aug 1;96(3):247-55. doi: 10.1016/j.bcp.2015.05.009. Epub 2015 May 29.PMID: 26032638
- ^ a b c d O'Flaherty JT, Rogers LC, Paumi CM, Hantgan RR, Thomas LR, Clay CE, High K, Chen YQ, Willingham MC, Smitherman PK, Kute TE, Rao A, Cramer SD, Morrow CS (Oct 2005). "5-Oxo-ETE analogs and the proliferation of cancer cells". Biochimica Et Biophysica Acta. 1736 (3): 228–236. doi:10.1016/j.bbalip.2005.08.009. PMID 16154383.
- ^ J Allergy Clin Immunol. 2005 Nov;116(5):1014-9.
- ^ Int Immunol. 2006 Nov;18(11):1575-83. Epub 2006 Sep 19
- ^ J Immunol. 1996 Nov 15;157(10):4664-71
- ^ J Immunol. 1996 Jul 1;157(1):336-42
- ^ J Biol Chem. 2004 Jul 2;279(27):28159-64
- ^ Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Review
- ^ J Allergy Clin Immunol. 2003 Oct;112(4):768-74
- ^ Am J Physiol Lung Cell Mol Physiol. 2004 Oct;287(4):L631-40
- ^ Prostaglandins Other Lipid Mediat. 2007 Jan;82(1-4):30-41
- ^ Ghosh J, Myers CE (Oct 1998). "Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells". Proceedings of the National Academy of Sciences of the United States of America. 95 (22): 13182–13187. Bibcode:1998PNAS...9513182G. doi:10.1073/pnas.95.22.13182. PMC 23752. PMID 9789062.
- ^ Rodríguez-Blanco G, Burgers PC, Dekker LJ, Ijzermans JJ, Wildhagen MF, Schenk-Braat EA, Bangma CH, Jenster G, Luider TM (May 2014). "Serum levels of arachidonic acid metabolites change during prostate cancer progression". The Prostate. 74 (6): 618–627. doi:10.1002/pros.22779. PMID 24435810.
- ^ Grant GE, Rubino S, Gravel S, Wang X, Patel P, Rokach J, Powell WS (Jun 2011). "Enhanced formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by cancer cells in response to oxidative stress, docosahexaenoic acid and neutrophil-derived 5-hydroxy-6,8,11,14-eicosatetraenoic acid". Carcinogenesis. 32 (6): 822–828. doi:10.1093/carcin/bgr044. PMC 3146358. PMID 21393477.
- ^ Avis IM, Jett M, Boyle T, Vos MD, Moody T, Treston AM, Martínez A, Mulshine JL (Feb 1996). "Growth control of lung cancer by interruption of 5-lipoxygenase-mediated growth factor signaling". The Journal of Clinical Investigation. 97 (3): 806–813. doi:10.1172/JCI118480. PMC 507119. PMID 8609238.
- ^ Biomed. Chromatogr. 8: 817–821. 2009.
{{cite journal}}: CS1 maint: untitled periodical (link) - ^ clin. Can. Res. 13:5736-5744, 200, Epub ahead of print
- ^ Hussey HJ, Tisdale MJ (Sep 1996). "Inhibition of tumour growth by lipoxygenase inhibitors". British Journal of Cancer. 74 (5): 683–687. doi:10.1038/bjc.1996.422. PMC 2074717. PMID 8795568.
- ^ Ding XZ, Iversen P, Cluck MW, Knezetic JA, Adrian TE (Jul 1999). "Lipoxygenase inhibitors abolish proliferation of human pancreatic cancer cells". Biochemical and Biophysical Research Communications. 261 (1): 218–213. doi:10.1006/bbrc.1999.1012. PMID 10405349.
- ^ Mol. Cell. Endocrin. 371:71-78, 2013
- ^ J Pharmacol Exp Ther. 1999 Nov;291(2):511-6
