Carbon sink

A carbon sink is anything, natural or otherwise, that accumulates and stores some carbon-containing chemical compound for an indefinite period and thereby removes carbon dioxide (CO2) from the atmosphere.[1]
Globally, the two most important carbon sinks are vegetation and the ocean.[2] Public awareness of the significance of CO2 sinks has grown since passage of the 1997 Kyoto Protocol, which promotes their use as a form of carbon offset.[3] There are also different strategies used to enhance this process. Soil is an important carbon storage medium. Much of the organic carbon retained in the soil of agricultural areas has been depleted due to intensive farming. "Blue carbon" designates carbon that is fixed via the ocean ecosystems. Mangroves, salt marshes and seagrasses make up a majority of ocean plant life and store large quantities of carbon.
Many efforts are being made to enhance natural sequestration in soils and the oceans.[1] In addition, a range of artificial sequestration initiatives are underway such as changed building construction materials, carbon capture and storage and geological sequestration.[4][5]
General

Increase in atmospheric carbon dioxide means increase in global temperature. The amount of carbon dioxide varies naturally in a dynamic equilibrium with photosynthesis of land plants. The natural sinks are:
- Soil is a carbon store and active carbon sink.[6]
- Photosynthesis by terrestrial plants with grass and trees allows them to serve as carbon sinks during growing seasons.
- Absorption of carbon dioxide by the oceans via solubility and biological pumps
While the creation of artificial sinks has been discussed, no major artificial systems remove carbon from the atmosphere on a material scale yet.[7]
Carbon sources include the combustion of fossil fuels (coal, natural gas, and oil) by humans for energy and transportation.[8]
Kyoto Protocol
The Kyoto Protocol was an international agreement that aimed at reducing carbon dioxide (CO2) emissions and the presence of greenhouse gases (GHG) in the atmosphere. The essential tenet of the Kyoto Protocol was that industrialized nations needed to reduce their CO2 emissions. Because growing vegetation takes in carbon dioxide, the Kyoto Protocol allows Annex I countries with large areas of growing forests to issue Removal Units to recognize the sequestration of carbon. The additional units make it easier for them to achieve their target emission levels. It is estimated that forests absorb between 10 to 20 tonnes per hectare (4.0 to 8.0 long ton/acre; 4.5 to 8.9 short ton/acre) each year, through photosynthetic conversion into starch, cellulose, lignin, and other components of wooden biomass. While this has been well documented for temperate forests and plantations, the fauna of the tropical forests place some limitations for such global estimates.[9]
Some countries seek to trade emission rights in carbon emission markets, purchasing the unused carbon emission allowances of other countries. If overall limits on greenhouse gas emission are put into place, cap and trade market mechanisms are purported to find cost-effective ways to reduce emissions.[10] There is as yet no carbon audit regime for all such markets globally, and none is specified in the Kyoto Protocol. National carbon emissions are self-declared.
In the Clean Development Mechanism, only afforestation and reforestation are eligible to produce certified emission reductions (CERs) in the first commitment period of the Kyoto Protocol (2008–2012). Forest conservation activities or activities avoiding deforestation, which would result in emission reduction through the conservation of existing carbon stocks, are not eligible at this time.[11] Also, agricultural carbon sequestration is not possible yet.[12]
Storage in terrestrial and marine environments
| Part of a series on the |
| Carbon cycle |
|---|
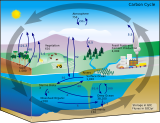 |
Soils
This section needs to be updated. The reason given is: IPCC Climate Change and Land report. (November 2019) |
Soils represent a short to long-term carbon storage medium, and contain more carbon than all terrestrial vegetation and the atmosphere combined.[13][14][15] Plant litter and other biomass including charcoal accumulates as organic matter in soils, and is degraded by chemical weathering and biological degradation. More recalcitrant organic carbon polymers such as cellulose, hemi-cellulose, lignin, aliphatic compounds, waxes and terpenoids are collectively retained as humus.[16] Organic matter tends to accumulate in litter and soils of colder regions such as the boreal forests of North America and the Taiga of Russia. Leaf litter and humus are rapidly oxidized and poorly retained in sub-tropical and tropical climate conditions due to high temperatures and extensive leaching by rainfall. Areas where shifting cultivation or slash and burn agriculture are practiced are generally only fertile for two to three years before they are abandoned. These tropical jungles are similar to coral reefs in that they are highly efficient at conserving and circulating necessary nutrients, which explains their lushness in a nutrient desert.[17] Much organic carbon retained in many agricultural areas worldwide has been severely depleted due to intensive farming practices.[18]
Grasslands contribute to soil organic matter, stored mainly in their extensive fibrous root mats. Due in part to the climatic conditions of these regions (e.g. cooler temperatures and semi-arid to arid conditions), these soils can accumulate significant quantities of organic matter. This can vary based on rainfall, the length of the winter season, and the frequency of naturally occurring lightning-induced grass-fires. While these fires release carbon dioxide, they improve the quality of the grasslands overall, in turn increasing the amount of carbon retained in the humic material. They also deposit carbon directly to the soil in the form of Biochar that does not significantly degrade back to carbon dioxide.[19]
Forest fires release absorbed carbon back into the atmosphere,[20] as does deforestation due to rapidly increased oxidation of soil organic matter.[21]
Organic matter in peat bogs undergoes slow anaerobic decomposition below the surface. This process is slow enough that in many cases the bog grows rapidly and fixes more carbon from the atmosphere than is released. Over time, the peat grows deeper. Peat bogs hold approximately one-quarter of the carbon stored in land plants and soils.[22]
Under some conditions, forests and peat bogs may become sources of CO2, such as when a forest is flooded by the construction of a hydroelectric dam. Unless the forests and peat are harvested before flooding, the rotting vegetation is a source of CO2 and methane comparable in magnitude to the amount of carbon released by a fossil-fuel powered plant of equivalent power.[23]
Regenerative agriculture
Current agricultural practices lead to carbon loss from soils. It has been suggested that improved farming practices could improve the capacity of the soil carbon sponge to hold carbon and water. Present worldwide practises of overgrazing are substantially reducing many grasslands' performance as soil carbon sponges.[24] The Rodale Institute says that regenerative agriculture, if practiced on the planet's tillable land of 15 million km2 (3.6 billion acres), could sequester up to 40% of current CO2 emissions.[25] They claim that agricultural carbon sequestration has the potential to mitigate global warming. When using biologically based regenerative practices, this dramatic benefit can be accomplished with no decrease in yields or farmer profits.[26] Organically managed soils can convert carbon dioxide from a greenhouse gas into a food-producing asset.[18]
In 2006, U.S. carbon dioxide emissions, largely from fossil fuel combustion, were estimated at nearly 5.9 billion tonnes (6.5 billion short tons).[27] If a 220 tonnes per square kilometre (2,000 lb/acre) per year sequestration rate was achieved on all 1.76 million km2 (434 million acres) of cropland in the United States, nearly 1.5 billion t (1.6 billion short tons) of carbon dioxide would be sequestered per year, mitigating close to one quarter of the country's total fossil fuel emissions.[18]
Riverine transport

Terrestrial and marine ecosystems are chiefly connected through riverine transport, which acts as the main channel through which erosive terrestrially derived substances enter into oceanic systems. Material and energy exchanges between the terrestrial biosphere and the lithosphere as well as organic carbon fixation and oxidation processes together regulate ecosystem carbon and dioxygen (O2) pools.[28]
Riverine transport, being the main connective channel of these pools, will act to transport net primary productivity (primarily in the form of dissolved organic carbon (DOC) and particulate organic carbon (POC)) from terrestrial to oceanic systems.[29] During transport, part of DOC will rapidly return to the atmosphere through redox reactions, causing "carbon degassing" to occur between land-atmosphere storage layers.[30][31] The remaining DOC and dissolved inorganic carbon (DIC) are also exported to the ocean.[32][33][34] Currently (2015) inorganic and organic carbon export fluxes from global rivers to the ocean amount to 0.50–0.70 Pg C y−1 and 0.15–0.35 Pg C y−1 respectively.[33] On the other hand, POC can remain buried in sediment over an extensive period, and the annual global terrestrial to oceanic POC flux has been estimated at 0.20 (+0.13,-0.07) Gg C y−1.[35][28]
Oceans

Blue carbon is a concept within climate change mitigation that refers to "biologically driven carbon fluxes and storage in marine systems that are amenable to management".[37]: 2220 Most commonly, it refers to the role that tidal marshes, mangroves and seagrass meadows can play in carbon sequestration.[37]: 2220 These ecosystems can play an important role for climate change mitigation and ecosystem-based adaptation. However, when blue carbon ecosystems are degraded or lost, they release carbon back to the atmosphere, thereby adding to greenhouse gas emissions.[37]: 2220
The methods for blue carbon management fall into the category of "ocean-based biological carbon dioxide removal (CDR) methods".[38]: 764 They are a type of biological carbon fixation.
Scientists are looking for ways to further develop the blue carbon potential of ecosystems.[39] However, the long-term effectiveness of blue carbon as a carbon dioxide removal solution is under debate.[40][39][41]
The term deep blue carbon is also in use and refers to storing carbon in the deep ocean waters.[42]Enhancing natural sequestration
Forests
Forests can be carbon stores,[43][44][45] and they are carbon dioxide sinks when they are increasing in density or area. In Canada's boreal forests as much as 80% of the total carbon is stored in the soils as dead organic matter.[46] A 40-year study of African, Asian, and South American tropical forests by the University of Leeds showed that tropical forests absorb about 18% of all carbon dioxide added by fossil fuels. For the last three decades, the amount of carbon absorbed by the world's intact tropical forests has fallen, according to a study published in 2020 in the journal Nature.

The total carbon stock in forests decreased from 668 gigatonnes in 1990 to 662 gigatonnes in 2020.[48] However, another study finds that the leaf area index has increased globally since 1981, which was responsible for 12.4% of the accumulated terrestrial carbon sink from 1981 to 2016. The CO2 fertilization effect, on the other hand, was responsible for 47% of the sink, while climate change reduced the sink by 28.6%.[49]
In 2019 they took up a third less carbon than they did in the 1990s, due to higher temperatures, droughts and deforestation. The typical tropical forest may become a carbon source by the 2060s.[50] Truly mature tropical forests, by definition, grow rapidly, with each tree producing at least 10 new trees each year. Based on studies by FAO and UNEP, it has been estimated that Asian forests absorb about 5 tonnes of carbon dioxide per hectare each year. The global cooling effect of carbon sequestration by forests is partially counterbalanced in that reforestation can decrease the reflection of sunlight (albedo). Mid-to-high-latitude forests have a much lower albedo during snow seasons than flat ground, thus contributing to warming. Modeling that compares the effects of albedo differences between forests and grasslands suggests that expanding the land area of forests in temperate zones offers only a temporary cooling benefit.[51][52][53][54]
In the United States in 2004 (the most recent year for which EPA statistics[55] are available), forests sequestered 10.6% (637 megatonnes)[56] of the carbon dioxide released in the United States by the combustion of fossil fuels (coal, oil, and natural gas; 5,657 megatonnes[57]). Urban trees sequestered another 1.5% (88 megatonnes).[56] To further reduce U.S. carbon dioxide emissions by 7%, as stipulated by the Kyoto Protocol, would require the planting of "an area the size of Texas [8% of the area of Brazil] every 30 years".[58] Carbon offset programs are planting millions of fast-growing trees per year to reforest tropical lands, for as little as $0.10 per tree; over their typical 40-year lifetime, one million of these trees will fix 1 a million tons of carbon dioxide.[59][60] In Canada, reducing timber harvesting would have very little impact on carbon dioxide emissions because of the combination of harvest and stored carbon in manufactured wood products along with the regrowth of the harvested forests. Additionally, the amount of carbon released from harvesting is small compared to the amount of carbon lost each year to forest fires and other natural disturbances.[46]
The Intergovernmental Panel on Climate Change concluded that "a sustainable forest management strategy aimed at maintaining or increasing forest carbon stocks, while producing an annual sustained yield of timber fibre or energy from the forest, will generate the largest sustained mitigation benefit".[61] Sustainable management practices keep forests growing at a higher rate over a potentially longer period of time, thus providing net sequestration benefits in addition to those of unmanaged forests.[62]
Life expectancy of forests varies throughout the world, influenced by tree species, site conditions and natural disturbance patterns. In some forests, carbon may be stored for centuries, while in other forests, carbon is released with frequent stand replacing fires. Forests that are harvested prior to stand replacing events allow for the retention of carbon in manufactured forest products such as lumber.[63] However, only a portion of the carbon removed from logged forests ends up as durable goods and buildings. The remainder ends up as sawmill by-products such as pulp, paper and pallets, which often end with incineration (resulting in carbon release into the atmosphere) at the end of their lifecycle. For instance, of the 1,692 megatonnes of carbon harvested from forests in Oregon and Washington from 1900 to 1992, only 23% is in long-term storage in forest products.[64]
Oceans
| Part of a series on |
| Biogeochemical cycles |
|---|
 |
One way to increase the carbon sequestration efficiency of the oceans is to add micrometre-sized iron particles in the form of either hematite (iron oxide) or melanterite (iron sulfate) to certain regions of the ocean. This has the effect of stimulating growth of plankton. Iron is an important nutrient for phytoplankton, usually made available via upwelling along the continental shelves, inflows from rivers and streams, as well as deposition of dust suspended in the atmosphere. Natural sources of ocean iron have been declining in recent decades, contributing to an overall decline in ocean productivity.[65] Yet in the presence of iron nutrients plankton populations quickly grow, or 'bloom', expanding the base of biomass productivity throughout the region and removing significant quantities of CO2 from the atmosphere via photosynthesis. A test in 2002 in the Southern Ocean around Antarctica suggests that between 10,000 and 100,000 carbon atoms are sunk for each iron atom added to the water.[66] Application of iron nutrients in select parts of the oceans, at appropriate scales, could have the combined effect of restoring ocean productivity while at the same time mitigating the effects of human caused emissions of carbon dioxide to the atmosphere.[65]
Because the effect of periodic small scale phytoplankton blooms on ocean ecosystems is unclear, more studies would be helpful. Phytoplankton have a complex effect on cloud formation via the release of substances such as dimethyl sulfide (DMS) that are converted to sulfate aerosols in the atmosphere, providing cloud condensation nuclei, or CCN.[67]
Other nutrients such as nitrates, phosphates, and silica as well as iron may cause ocean fertilization. There has been some speculation that using pulses of fertilization (around 20 days in length) may be more effective at getting carbon to ocean floor than sustained fertilization.[68]
There is some controversy over seeding the oceans with iron however, due to the potential for increased toxic phytoplankton growth (e.g. "red tide"), declining water quality due to overgrowth, and increasing anoxia in areas harming other sea-life such as zooplankton, fish, coral, etc.[69][70]
Soils
Since the 1850s, a large proportion of the world's grasslands have been tilled and converted to croplands, allowing the rapid oxidation of large quantities of soil organic carbon. However, in the United States in 2004 (the most recent year for which EPA statistics are available), agricultural soils including pasture land sequestered 0.8% (46 megatonne)[56] as much carbon as was released in the United States by the combustion of fossil fuels (5,988 megatonne).[57] The annual amount of this sequestration has been gradually increasing since 1998.[71][56]
Methods that significantly enhance carbon sequestration in soil include no-till farming, residue mulching, cover cropping, and crop rotation, all of which are more widely used in organic farming than in conventional farming.[72][73] Because only 5% of US farmland currently uses no-till and residue mulching, there is a large potential for carbon sequestration.[74] Conversion to pastureland, particularly with good management of grazing, can sequester even more carbon in the soil.
Terra preta, an anthropogenic, high-carbon soil, is also being investigated as a sequestration mechanism. By pyrolysing biomass, about half of its carbon can be reduced to charcoal, which can persist in the soil for centuries, and makes a useful soil amendment, especially in tropical soils (biochar or agrichar).[75][76]
"For most of human history, permafrost has been Earth's largest terrestrial carbon sink, trapping plant and animal material in its frozen layers for centuries. It currently stores about 1,600 billion tonnes of carbon—more than twice the amount in the atmosphere today. But thanks to rising temperatures, permafrost is fracturing and disappearing".[77] Sergey Zimov has proposed to restore and protect this major carbon sequestration mechanism via restoration of grassland and large arctic mammalian herbivores.[78]
Savanna
Controlled burns on far north Australian savannas can result in an overall carbon sink. One working example is the West Arnhem Fire Management Agreement, started to bring "strategic fire management across 28,000 km2 of Western Arnhem Land". Deliberately starting controlled burns early in the dry season results in a mosaic of burnt and unburnt country which reduces the area of burning compared with stronger, late dry season fires. In the early dry season there are higher moisture levels, cooler temperatures, and lighter wind than later in the dry season; fires tend to go out overnight. Early controlled burns also results in a smaller proportion of the grass and tree biomass being burnt.[79] Emission reductions of 256,000 tonnes of CO2 have been made as of 2007.[80]
Artificial sequestration
For carbon to be sequestered artificially (i.e. not using the natural processes of the carbon cycle) it must first be captured, or it must be significantly delayed or prevented from being re-released into the atmosphere (by combustion, decay, etc.) from an existing carbon-rich material, by being incorporated into an enduring usage (such as in construction). Thereafter it can be passively stored or remain productively utilized over time in a variety of ways. For instance, upon harvesting, wood (as a carbon-rich material) can be immediately burned or otherwise serve as a fuel, returning its carbon to the atmosphere, or it can be incorporated into construction or a range of other durable products, thus sequestering its carbon over years or even centuries.[81]
A very carefully designed and durable, energy-efficient and energy-capturing building has the potential to sequester (in its carbon-rich construction materials), as much as or more carbon than was released by the acquisition and incorporation of all its materials and than will be released by building-function "energy-imports" during the structure's (potentially multi-century) existence. Such a structure might be termed "carbon neutral" or even "carbon negative". Building construction and operation (electricity usage, heating, etc.) are estimated to contribute nearly half of the annual human-caused carbon additions to the atmosphere.[82]
Natural-gas purification plants often already have to remove carbon dioxide, either to avoid dry ice clogging gas tankers or to prevent carbon-dioxide concentrations exceeding the 3% maximum permitted on the natural-gas distribution grid.[83]
Buildings

According to an international team of interdisciplinary scientists in a 2020 study, broad-base adoption of mass timber and their substitution for steel and concrete in new mid-rise construction projects over the next few decades has the potential to turn timber buildings into a global carbon sink, as they store the carbon dioxide taken up from the air by trees that are harvested and used as mass timber. Noting the demographic need for new urban construction for the next thirty years, the team analyzed four scenarios for the transition to mass-timber new mid-rise construction. Assuming business as usual, only 0.5% of new buildings worldwide would be constructed with timber by 2050 (scenario 1). This could be driven up to 10% (scenario 2) or 50% (scenario 3), assuming mass timber manufacturing would increase as a material revolution replacing cement and steel in urban construction by wood scales up accordingly. Lastly, if countries with current low industrialization level, e.g., Africa, Oceania, and parts of Asia, would also make the transition to timber (including bamboo), then even 90% timber by 2050 (scenario 4) is conceivable. This could result in storing between 10 million tons of carbon per year in the lowest scenario and close to 700 million tons in the highest scenario. The study found that this potential could be realized under two conditions. First, the harvested forests would need to be sustainably managed, governed, and used. Second, wood from demolished timber buildings would need to be reused or preserved on land in various forms.[84]
Direct air capture


Direct air capture (DAC) is the use of chemical or physical processes to extract carbon dioxide directly from the ambient air.[85] If the extracted CO2 is then sequestered in safe long-term storage (called direct air carbon capture and sequestration (DACCS), the overall process will achieve carbon dioxide removal and be a "negative emissions technology" (NET).
The carbon dioxide (CO2) is captured directly from the ambient air; this is contrast to carbon capture and storage (CCS) which captures CO2 from point sources, such as a cement factory or a bioenergy plant.[86] After the capture, DAC generates a concentrated stream of CO2 for sequestration or utilization. Carbon dioxide removal is achieved when ambient air makes contact with chemical media, typically an aqueous alkaline solvent[87] or sorbents.[88] These chemical media are subsequently stripped of CO2 through the application of energy (namely heat), resulting in a CO2 stream that can undergo dehydration and compression, while simultaneously regenerating the chemical media for reuse.
When combined with long-term storage of CO2, DAC is known as direct air carbon capture and storage (DACCS or DACS[89]). DACCS can function as both a carbon dioxide removal mechanism or a carbon negative technology. As of 2023, DACCS has yet to be integrated into emissions trading because, at over US$1000,[90] the cost per ton of carbon dioxide is many times the carbon price on those markets.[91] For the end-to-end process to remain net carbon negative, DAC machines must be powered by renewable energy sources, since the process can be quite energy expensive. Future innovations may reduce the energy intensity of this process.
DAC was suggested in 1999 and is still in development.[92][93] Several commercial plants are planned or in operation in Europe and the US. Large-scale DAC deployment may be accelerated when connected with economical applications or policy incentives.
In contrast to carbon capture and storage (CCS) which captures emissions from a point source such as a factory, DAC reduces the carbon dioxide concentration in the atmosphere as a whole. Thus, DAC can be used to capture emissions that originated in non-stationary sources such as airplanes.[86]Oceans
Another proposed form of carbon sequestration in the ocean is direct injection. In this method, carbon dioxide is pumped directly into the water at depth, and expected to form "lakes" of liquid CO2 at the bottom. Experiments carried out in moderate to deep waters (350–3,600 metres (1,150–11,810 ft)) indicate that the liquid CO2 reacts to form solid CO2 clathrate hydrates, which gradually dissolve in the surrounding waters.[94]
This method, too, has potentially dangerous environmental consequences. The carbon dioxide does react with the water to form carbonic acid, H2CO3; however, most (as much as 99%) remains as dissolved molecular CO2. The equilibrium would no doubt be quite different[vague] under the high pressure conditions in the deep ocean. In addition, if deep-sea bacterial methanogens that reduce carbon dioxide were to encounter the carbon dioxide sinks, levels of methane gas may increase, leading to the generation of an even worse greenhouse gas.[95] The resulting environmental effects on benthic life forms of the bathypelagic, abyssopelagic and hadopelagic zones are unknown. Even though life appears to be rather sparse in the deep ocean basins, energy and chemical effects in these deep basins could have far-reaching implications. Much more work is needed here to define the extent of the potential problems.
Carbon storage in or under oceans may not be compatible with the Convention on the Prevention of Marine Pollution by Dumping of Wastes and Other Matter.[96]
An additional method of long-term ocean-based sequestration is to gather crop residue such as corn stalks or excess hay into large weighted bales of biomass and deposit it in the alluvial fan areas of the deep ocean basin. Dropping these residues in alluvial fans would cause the residues to be quickly buried in silt on the sea floor, sequestering the biomass for very long time spans. Alluvial fans exist in all of the world's oceans and seas where river deltas fall off the edge of the continental shelf such as the Mississippi alluvial fan in the gulf of Mexico and the Nile alluvial fan in the Mediterranean Sea. A downside, however, would be an increase in aerobic bacteria growth due to the introduction of biomass, leading to more competition for oxygen resources in the deep sea, similar to the oxygen minimum zone.[97]
Geological sequestration
The method of geo-sequestration or geological storage involves injecting carbon dioxide directly into underground geological formations.[98] Declining oil fields, saline aquifers, and unmineable coal seams have been suggested as storage sites. Caverns and old mines that are commonly used to store natural gas are not considered, because of a lack of storage safety.
CO2 has been injected into declining oil fields for more than 40 years, to increase oil recovery. This option is attractive because the storage costs are offset by the sale of additional oil that is recovered. Typically, 10–15% additional recovery of the original oil in place is possible. Further benefits are the existing infrastructure and the geophysical and geological information about the oil field that is available from the oil exploration. Another benefit of injecting CO2 into oil fields is that CO2 is soluble in oil. Dissolving CO2 in oil lowers the viscosity of the oil and reduces its interfacial tension which increases the oils mobility. All oil fields have a geological barrier preventing upward migration of oil. As most oil and gas has been in place for millions to tens of millions of years, depleted oil and gas reservoirs can contain carbon dioxide for millennia. Identified possible problems are the many 'leak' opportunities provided by old oil wells, the need for high injection pressures and acidification which can damage the geological barrier. Other disadvantages of old oil fields are their limited geographic distribution and depths, which require high injection pressures for sequestration. Below a depth of about 1000 m, carbon dioxide is injected as a supercritical fluid, a material with the density of a liquid, but the viscosity and diffusivity of a gas. Unmineable coal seams can be used to store CO2, because CO2 absorbs to the coal surface, ensuring safe long-term storage. In the process it releases methane that was previously adsorbed to the coal surface and that may be recovered. Again the sale of the methane can be used to offset the cost of the CO2 storage. Release or burning of methane would of course at least partially offset the obtained sequestration result – except when the gas is allowed to escape into the atmosphere in significant quantities: methane has a 80-fold higher global warming potential than CO2 (during the first twenty years).[99]
Saline aquifers contain highly mineralized brines and have so far been considered of no benefit to humans except in a few cases where they have been used for the storage of chemical waste. Their advantages include a large potential storage volume and relatively common occurrence reducing the distance over which CO2 has to be transported. The major disadvantage of saline aquifers is that relatively little is known about them compared to oil fields. Another disadvantage of saline aquifers is that as the salinity of the water increases, less CO2 can be dissolved into aqueous solution. To keep the cost of storage acceptable the geophysical exploration may be limited, resulting in larger uncertainty about the structure of a given aquifer. Unlike storage in oil fields or coal beds, no side product will offset the storage cost. Leakage of CO2 back into the atmosphere may be a problem in saline-aquifer storage. However, current research shows that several trapping mechanisms immobilize the CO2 underground, reducing the risk of leakage.[100]
A major research project examining the geological sequestration of carbon dioxide is currently being performed at an oil field at Weyburn in south-eastern Saskatchewan. In the North Sea, Norway's Equinor natural-gas platform Sleipner strips carbon dioxide out of the natural gas with amine solvents and disposes of this carbon dioxide by geological sequestration. Sleipner reduces emissions of carbon dioxide by approximately one million tonnes a year. The cost of geological sequestration is minor relative to the overall running costs. One of the first planned trial of large-scale sequestration of carbon dioxide stripped from power plant emissions in the Miller oilfield as its reserves are depleted by BP was not funded.[101]
In October 2007, the Bureau of Economic Geology at The University of Texas at Austin received a 10-year, $38 million subcontract to conduct the first intensively monitored, long-term project in the United States studying the feasibility of injecting a large volume of CO2 for underground storage.[102] The project is a research program of the Southeast Regional Carbon Sequestration Partnership (SECARB), funded by the National Energy Technology Laboratory of the U.S. Department of Energy (DOE). The SECARB partnership will demonstrate CO2 injection rate and storage capacity in the Tuscaloosa-Woodbine geologic system that stretches from Texas to Florida. Beginning in fall 2007, the project will inject CO2 at the rate of one million tons[vague] per year, for up to 1.5 years, into brine up to 10,000 feet (3,000 m) below the land surface near the Cranfield oil field about 15 miles (24 km) east of Natchez, Mississippi. Experimental equipment will measure the ability of the subsurface to accept and retain CO2.[94]
Mineral sequestration
Mineral sequestration aims to trap carbon in the form of solid carbonate salts. This process occurs slowly in nature and is responsible for the deposition and accumulation of limestone over geologic time. Carbonic acid in groundwater slowly reacts with complex silicates to dissolve calcium, magnesium, alkalis and silica and leave a residue of clay minerals. The dissolved calcium and magnesium react with bicarbonate to precipitate calcium and magnesium carbonates, a process that organisms use to make shells. When the organisms die, their shells are deposited as sediment and eventually turn into limestone. Limestones have accumulated over billions of years of geologic time and contain much of Earth's carbon. Ongoing research aims to speed up similar reactions involving alkali carbonates.[103]
Several serpentinite deposits are being investigated as potentially large scale CO2 storage sinks such as those found in NSW, Australia, where the first mineral carbonation pilot plant project is underway.[104] Beneficial re-use of magnesium carbonate from this process could provide feedstock for new products developed for the built environment and agriculture without returning the carbon into the atmosphere and so acting as a carbon sink.[105]
One proposed reaction is that of the olivine-rich rock dunite, or its hydrated equivalent serpentinite with carbon dioxide to form the carbonate mineral magnesite, plus silica and iron oxide (magnetite).
Serpentinite sequestration is favored because of the non-toxic and stable nature of magnesium carbonate. The ideal reactions involve the magnesium endmember components of the olivine (reaction 1) or serpentine (reaction 2), the latter derived from earlier olivine by hydration and silicification (reaction 3). The presence of iron in the olivine or serpentine reduces the efficiency of sequestration, since the iron components of these minerals break down to iron oxide and silica (reaction 4).
Serpentinite reactions
| + → + + | (Reaction 1) |
| + → + + | (Reaction 2) |
| + + → | (Reaction 3) |
| + → + + | (Reaction 4) |
Zeolitic imidazolate frameworks
Zeolitic imidazolate frameworks is a metal-organic framework carbon dioxide sink which could be used to keep industrial emissions of carbon dioxide out of the atmosphere.[106]
Trends in sink performance

One study in 2009 found that the fraction of fossil-fuel emissions absorbed by the oceans may have declined by up to 10% since 2000, indicating oceanic sequestration may be sublinear.[108] Another 2009 study found that the fraction of CO2 absorbed by terrestrial ecosystems and the oceans has not changed since 1850, indicating undiminished capacity.[109]
One study in 2020 found that 32 tracked Brazilian non-Amazon seasonal tropical forests declined from a carbon sink to a carbon source in 2013 and concludes that "policies are needed to mitigate the emission of greenhouse gases and to restore and protect tropical seasonal forests".[110][111]
The IPCC has noted that oceans and vegetation will progressively absorb a smaller fraction of CO2 emissions and, in return, create a larger absorption shortcoming.[112]
An emerging trend is the use of conservative or regenerative agriculture. According to Project Drawdown, regenerative agriculture could sink 9.43 to 13.4 gigatons of CO2 between 2020 and 2050. This will be a huge contribution to sink performance.[113]
See also
- Azolla event
- Biochar
- Bio-energy with carbon capture and storage
- Carbon capture and storage
- Carbon cycle
- Carbon sequestration in terrestrial ecosystems
- Carbon sinks and removal for climate change mitigation
- Fluxnet-Canada Research Network, research initiative on post forest disturbance carbon sinking
Sources
![]() This article incorporates text from a free content work. Licensed under CC BY-SA 3.0 IGO (license statement/permission). Text taken from Global Forest Resources Assessment 2020 Key findings, FAO, FAO.
This article incorporates text from a free content work. Licensed under CC BY-SA 3.0 IGO (license statement/permission). Text taken from Global Forest Resources Assessment 2020 Key findings, FAO, FAO.
References
- ^ a b "What is a carbon sink?". www.clientearth.org. Retrieved 18 June 2021.
- ^ "Carbon Sources and Sinks". National Geographic Society. 26 March 2020. Archived from the original on 14 December 2020. Retrieved 18 June 2021.
- ^ "carbon sink — European Environment Agency". www.eea.europa.eu. Retrieved 18 June 2021.
- ^ Churkina, Galina; Organschi, Alan; Reyer, Christopher P. O.; Ruff, Andrew; Vinke, Kira; Liu, Zhu; Reck, Barbara K.; Graedel, T. E.; Schellnhuber, Hans Joachim (2020). "Buildings as a global carbon sink". Nature Sustainability. 3 (4): 269–276. doi:10.1038/s41893-019-0462-4. ISSN 2398-9629. S2CID 213032074.
- ^ "carbon sequestration | Definition, Methods, & Climate Change". Encyclopedia Britannica. Retrieved 18 June 2021.
- ^ Blakemore, R.J. (2018). "Non-Flat Earth Recalibrated for Terrain and Topsoil". Soil Systems. 2 (4): 64. doi:10.3390/soilsystems2040064.
- ^ "Carbon Sinks: A Brief Review". Earth.Org - Past | Present | Future. Retrieved 2 December 2020.
- ^ Environmental Protection Agency, United States (12 August 2013). "Overview of Greenhouse Gases". EPA Climate Change. US EPA. Retrieved 17 May 2015.
- ^ "STATE OF THE WORLD'S FORESTS 2001". www.fao.org. Retrieved 18 June 2021.
- ^ Karen Palmer; Dallas Burtraw. "Electricity, Renewables, and Climate Change: Searching for a Cost-Effective Policy" (PDF). Resources for the Future. Archived from the original (PDF) on 4 June 2007.
- ^ Manguiat MSZ, Verheyen R, Mackensen J, Scholz G (2005). "Legal aspects in the implementation of CDM forestry projects" (PDF). IUCN Environmental Policy and Law Papers. Number 59. Archived from the original (PDF) on 16 July 2010.
- ^ Rosenbaum KL, Schoene D, Mekouar A (2004). "Climate change and the forest sector. Possible national and subnational legislation". FAO Forestry Papers. Number 144.
- ^ Swift, Roger S. (November 2001). "Sequestration of Carbon by soil". Soil Science. 166 (11): 858–71. Bibcode:2001SoilS.166..858S. doi:10.1097/00010694-200111000-00010. S2CID 96820247.
- ^ Batjes, Niels H. (1996). "Total carbon and nitrogen in the soils of the world". European Journal of Soil Science. 47 (2): 151–63. doi:10.1111/j.1365-2389.1996.tb01386.x.
- ^ Batjes (2016). "Harmonised soil property values for broad-scale modelling (WISE30sec) with estimates of global soil carbon stocks". Geoderma. 269: 61–68. Bibcode:2016Geode.269...61B. doi:10.1016/j.geoderma.2016.01.034.
- ^ Klaus Lorenza; Rattan Lala; Caroline M. Prestonb; Klaas G.J. Nieropc (15 November 2007). "Strengthening the soil organic carbon pool by increasing contributions from recalcitrant aliphatic bio(macro)molecules". Geoderma. 142 (1–2): 1–10. Bibcode:2007Geode.142....1L. doi:10.1016/j.geoderma.2007.07.013.
- ^ "Coral Reefs Biome "Underwater Rainforests"". Retrieved 19 September 2021.
- ^ a b c "Organic Farming Can Cool the World that Chemical Farming Overheated". 17 October 2009. Retrieved 18 September 2021.
- ^ Woolf, Dominic; Amonette, James E.; Street-Perrott, F. Alayne; Lehmann, Johannes; Joseph, Stephen (10 August 2010). "Sustainable biochar to mitigate global climate change". Nature Communications. 1 (5): 56. Bibcode:2010NatCo...1...56W. doi:10.1038/ncomms1053. ISSN 2041-1723. PMC 2964457. PMID 20975722.
- ^ Mooney, Chris. "The really scary thing about wildfires is how they can worsen climate change". The Washington Post. Retrieved 24 January 2017.
- ^ "Deforestation & Carbon Emission". Consulting Geologist. Retrieved 24 January 2017.
- ^ Chester, Bronwyn (20 April 2000). "The case of the missing sink". McGill Reporter. Retrieved 17 June 2022.
- ^ Duncan Graham-Rowe (24 February 2005). "Hydroelectric power's dirty secret revealed". New Scientist. Archived from the original on 18 May 2008. Retrieved 8 July 2008.
- ^ C. Michael Hogan (28 December 2009). "Overgrazing". In Cutler J. Cleveland (ed.). Encyclopedia of Earth. Sidney Draggan (Topic Editor). Washington DC: Environmental Information Coalition, National Council for Science and the Environment. Archived from the original on 11 July 2010.
- ^ Timothy J. LaSalle; Paul Hepperly (2008). Regenerative 21st Century Farming: A Solution to Global Warming (PDF) (Report). The Rodale Institute. Archived from the original (PDF) on 10 September 2008. Retrieved 19 May 2008.
- ^ "The Farming Systems Trial" (PDF). Rodale Institute. Archived from the original (PDF) on 22 February 2013. Retrieved 20 November 2013.
- ^ "Carbon Dioxide and Our Ocean Legacy, by Richard A. Feely et. al" (PDF).
- ^ a b c Gao, Yang; Jia, Junjie; Lu, Yao; Sun, Kun; Wang, Jing; Wang, Shuoyue (2022). "Carbon transportation, transformation, and sedimentation processes at the land-river-estuary continuum". Fundamental Research. Elsevier BV. doi:10.1016/j.fmre.2022.07.007. ISSN 2667-3258.
 Modified material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Schlünz, B.; Schneider, R. R. (22 March 2000). "Transport of terrestrial organic carbon to the oceans by rivers: re-estimating flux- and burial rates". International Journal of Earth Sciences. 88 (4). Springer Science and Business Media LLC: 599–606. doi:10.1007/s005310050290. ISSN 1437-3254.
- ^ 10.1016/j.marchem.2004.06.023
- ^ Bouchez, Julien; Beyssac, Olivier; Galy, Valier; Gaillardet, Jérôme; France-Lanord, Christian; Maurice, Laurence; Moreira-Turcq, Patricia (2010). "Oxidation of petrogenic organic carbon in the Amazon floodplain as a source of atmospheric CO2". Geology. 38 (3). Geological Society of America: 255–258. doi:10.1130/g30608.1. ISSN 1943-2682.
- ^ Regnier, Pierre; Friedlingstein, Pierre; Ciais, Philippe; Mackenzie, Fred T.; et al. (9 June 2013). "Anthropogenic perturbation of the carbon fluxes from land to ocean". Nature Geoscience. 6 (8). Springer Science and Business Media LLC: 597–607. doi:10.1038/ngeo1830. ISSN 1752-0894.
- ^ a b Bauer, James E.; Cai, Wei-Jun; Raymond, Peter A.; Bianchi, Thomas S.; Hopkinson, Charles S.; Regnier, Pierre A. G. (4 December 2013). "The changing carbon cycle of the coastal ocean". Nature. 504 (7478). Springer Science and Business Media LLC: 61–70. doi:10.1038/nature12857. ISSN 0028-0836.
- ^ Cai, Wei-Jun (15 January 2011). "Estuarine and Coastal Ocean Carbon Paradox: CO2 Sinks or Sites of Terrestrial Carbon Incineration?". Annual Review of Marine Science. 3 (1). Annual Reviews: 123–145. doi:10.1146/annurev-marine-120709-142723. ISSN 1941-1405.
- ^ Galy, Valier; Peucker-Ehrenbrink, Bernhard; Eglinton, Timothy (2015). "Global carbon export from the terrestrial biosphere controlled by erosion". Nature. 521 (7551). Springer Science and Business Media LLC: 204–207. doi:10.1038/nature14400. ISSN 0028-0836.
- ^ Huxham, M.; Whitlock, D.; Githaiga, M.; Dencer-Brown, A. (2018). "Carbon in the Coastal Seascape: How Interactions Between Mangrove Forests, Seagrass Meadows and Tidal Marshes Influence Carbon Storage". Current Forestry Reports. 4 (2): 101–110. Bibcode:2018CForR...4..101H. doi:10.1007/s40725-018-0077-4. S2CID 135243725.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Archived 2017-10-16 at the Wayback Machine.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Archived 2017-10-16 at the Wayback Machine.
- ^ a b c IPCC, 2021: Annex VII: Glossary [Matthews, J. B. R., V. Möller, R. van Diemen, J. S. Fuglestvedt, V. Masson-Delmotte, C. Méndez, S. Semenov, A. Reisinger (eds.)]. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 2215–2256, doi:10.1017/9781009157896.022.
- ^ Canadell, J. G., P. M. S. Monteiro, M. H. Costa, L. Cotrim da Cunha, P. M. Cox, A. V. Eliseev, S. Henson, M. Ishii, S. Jaccard, C. Koven, A. Lohila, P. K. Patra, S. Piao, J. Rogelj, S. Syampungani, S. Zaehle, and K. Zickfeld, 2021: Chapter 5: Global Carbon and other Biogeochemical Cycles and Feedbacks. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 673–816, doi:10.1017/9781009157896.007.
- ^ a b Ricart, Aurora M.; Krause-Jensen, Dorte; Hancke, Kasper; Price, Nichole N.; Masqué, Pere; Duarte, Carlos M. (2022). "Sinking seaweed in the deep ocean for carbon neutrality is ahead of science and beyond the ethics". Environmental Research Letters. 17 (8): 081003. Bibcode:2022ERL....17h1003R. doi:10.1088/1748-9326/ac82ff. hdl:10754/679874. S2CID 250973225.
- ^ Hurd, Catriona L.; Law, Cliff S.; Bach, Lennart T.; Britton, Damon; Hovenden, Mark; Paine, Ellie R.; Raven, John A.; Tamsitt, Veronica; Boyd, Philip W. (2022). "Forensic carbon accounting: Assessing the role of seaweeds for carbon sequestration". Journal of Phycology. 58 (3): 347–363. Bibcode:2022JPcgy..58..347H. doi:10.1111/jpy.13249. PMID 35286717. S2CID 247453370.
- ^ Boyd, Philip W.; Bach, Lennart T.; Hurd, Catriona L.; Paine, Ellie; Raven, John A.; Tamsitt, Veronica (2022). "Potential negative effects of ocean afforestation on offshore ecosystems". Nature Ecology & Evolution. 6 (6): 675–683. Bibcode:2022NatEE...6..675B. doi:10.1038/s41559-022-01722-1. PMID 35449458. S2CID 248322820.
- ^ "What Is Blue Carbon?". CarbonBetter. 4 November 2022. Retrieved 20 May 2023.
- ^ Carolyn Gramling (28 September 2017). "Tropical forests have flipped from sponges to sources of carbon dioxide; A closer look at the world's trees reveals a loss of density in the tropics". Sciencenews.org. 358 (6360): 230–234. Bibcode:2017Sci...358..230B. doi:10.1126/science.aam5962. PMID 28971966. Retrieved 6 October 2017.
- ^ Baccini A, Walker W, Carvalho L, Farina M, Sulla-Menashe D, Houghton RA (13 October 2017). "Tropical forests are a net carbon source based on aboveground measurements of gain and loss". Science. 358 (6360): 230–234. Bibcode:2017Sci...358..230B. doi:10.1126/science.aam5962. PMID 28971966.
- ^ Spawn, Seth A.; Sullivan, Clare C.; Lark, Tyler J.; Gibbs, Holly K. (December 2020). "Harmonized global maps of above and belowground biomass carbon density in the year 2010". Scientific Data. 7 (1): 112. Bibcode:2020NatSD...7..112S. doi:10.1038/s41597-020-0444-4. PMC 7136222. PMID 32249772.
- ^ a b "Does harvesting in Canada's forests contribute to climate change?" (PDF). Canadian Forest Service Science-Policy Notes. Natural Resources Canada. May 2007.[permanent dead link]
- ^ Global Forest Resources Assessment 2020 – Key findings. Rome: FAO. 2020. doi:10.4060/ca8753en. ISBN 978-92-5-132581-0. S2CID 130116768.
- ^ Global Forest Resources Assessment 2020 – Key findings. FAO. 2020. doi:10.4060/ca8753en. ISBN 978-92-5-132581-0. S2CID 130116768.
- ^ Chen, JM; Ciais, Philippe (18 September 2019). "Vegetation structural change since 1981 significantly enhanced the terrestrial carbon sink". Nature Communications. 10 (4259): 4259. Bibcode:2019NatCo..10.4259C. doi:10.1038/s41467-019-12257-8. PMC 6751163. PMID 31534135.
- ^ Harvey, Fiona (4 March 2020). "Tropical forests losing their ability to absorb carbon, study finds". The Guardian. ISSN 0261-3077. Retrieved 5 March 2020.
- ^ Jonathan Amos (15 December 2006). "Care needed with carbon offsets". BBC. Retrieved 8 July 2008.
- ^ "Models show growing more forests in temperate regions could contribute to global warming". Lawrence Livermore National Laboratory. 5 December 2005. Archived from the original on 27 May 2010. Retrieved 8 July 2008.
- ^ S. Gibbard; K. Caldeira; G. Bala; T. J. Phillips; M. Wickett (December 2005). "Climate effects of global land cover change". Geophysical Research Letters. 32 (23): L23705. Bibcode:2005GeoRL..3223705G. doi:10.1029/2005GL024550.
- ^ Malhi, Yadvinder; Meir, Patrick; Brown, Sandra (2002). "Forests, carbon and global climate". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 360 (1797): 1567–91. Bibcode:2002RSPTA.360.1567M. doi:10.1098/rsta.2002.1020. PMID 12460485. S2CID 1864078.
- ^ "U.S. Greenhouse Gas Inventory Reports". EPA. Archived from the original on 1 June 2010. Retrieved 8 July 2008.
- ^ a b c d "Land Use, Land-Use Change, and Forestry" (PDF). EPA. Archived from the original (PDF) on 23 May 2008. Retrieved 8 July 2008.
- ^ a b "Executive Summary" (PDF). EPA. Archived from the original (PDF) on 18 July 2008. Retrieved 8 July 2008.
- ^ William H. Schlesinger, dean of the Nicholas School of the Environment and Earth Sciences at Duke University, in Durham, North Carolina.
- ^ "This Is The Impact Of 1 Million Trees". 26 November 2019. Retrieved 18 September 2021.
- ^ Grant M. Domke; Sonja N. Oswalt; Brian F. Walters; Randall S. Morin (6 October 2020). "Tree planting has the potential to increase carbon sequestration capacity of forests in the United States" (PDF). PNAS. 117 (40): 24649–24651. Bibcode:2020PNAS..11724649D. doi:10.1073/pnas.2010840117. PMC 7547226. PMID 32958649. S2CID 221842058.
- ^ "Fourth Assessment Report (AR4): Mitigation of Climate Change (Working Group III)" (PDF). International Panel on Climate Change. p. 549. Archived from the original (PDF) on 4 August 2009. Retrieved 11 August 2009.
- ^ Ruddell, Steven; et al. (September 2007). "The Role for Sustainably Managed Forests in Climate Change Mitigation". Journal of Forestry. 105 (6): 314–319.
- ^ J. Chatellier (January 2010). The Role of Forest Products in the Global Carbon Cycle: From In-Use to End-of-Life (PDF). Yale School of Forestry and Environmental Studies. Archived from the original (PDF) on 5 July 2010.
- ^ Harmon, M. E.; Harmon, J. M.; Ferrell, W. K.; Brooks, D. (1996). "Modeling carbon stores in Oregon and Washington forest products: 1900?1992". Climatic Change. 33 (4): 521. Bibcode:1996ClCh...33..521H. doi:10.1007/BF00141703. S2CID 27637103.
- ^ a b David E. Steitz; Krishna Ramanujan; Kent LaBorde (2003). "Ocean Plant Life Slows Down And Absorbs Less Carbon".
- ^ Quirin Schiermeier (22 April 2004). "Iron seeding creates fleeting carbon sink in Southern Ocean". Nature. 428 (6985): 788. Bibcode:2004Natur.428..788S. doi:10.1038/428788b. PMID 15103342. S2CID 33727485.
- ^ Roelofs, G. (2008). "A GCM study of organic matter in marine aerosol and its potential contribution to cloud drop activation". Atmospheric Chemistry and Physics. 8 (3): 709–719. Bibcode:2008ACP.....8..709R. doi:10.5194/acp-8-709-2008. hdl:2066/34516.
- ^ Michael Markels Jr; Richard T. Barber (14–17 May 2001). "Sequestration of CO2 by ocean fertilization" (PDF). NETL Conference on Carbon Sequestration. Archived from the original (PDF) on 10 September 2008. Retrieved 8 July 2008.
- ^ "Questions and Concerns". GreenSea Venture. Archived from the original on 15 April 2008. Retrieved 8 July 2008.
- ^ Mitrovic, Simon M.; Fernández Amandi, Monica; McKenzie, Lincoln; Furey, Ambrose; James, Kevin J. (2004). "Effects of selenium, iron and cobalt addition to growth and yessotoxin production of the toxic marine dinoflagellate Protoceratium reticulatum in culture". Journal of Experimental Marine Biology and Ecology. 313 (2): 337–51. doi:10.1016/j.jembe.2004.08.014.
- ^ "Visualizing Carbon Storage in Earth's Ecosystems". 25 January 2022. Retrieved 31 January 2022.
- ^ Susan S. Lang (13 July 2005). "Organic farming produces same corn and soybean yields as conventional farms, but consumes less energy and no pesticides, study finds". Retrieved 8 July 2008.
- ^ Pimentel, David; Hepperly, Paul; Hanson, James; Douds, David; Seidel, Rita (2005). "Environmental, Energetic, and Economic Comparisons of Organic and Conventional Farming Systems". BioScience. 55 (7): 573–82. doi:10.1641/0006-3568(2005)055[0573:EEAECO]2.0.CO;2.
- ^ Lal, Rattan; Griffin, Michael; Apt, Jay; Lave, Lester; Morgan, M. Granger (2004). "Ecology: Managing Soil Carbon". Science. 304 (5669): 393. doi:10.1126/science.1093079. PMID 15087532. S2CID 129925989.
- ^ Johannes Lehmann. "Biochar: the new frontier". Archived from the original on 18 June 2008. Retrieved 8 July 2008.
- ^ Horstman, Mark (23 September 2007). "Agrichar – A solution to global warming?". ABC TV Science: Catalyst. Australian Broadcasting Corporation. Retrieved 8 July 2008.
- ^ Monique Brouillette (17 March 2021). "How microbes in permafrost could trigger a massive carbon bomb". Nature. 591 (7850): 360–362. Bibcode:2021Natur.591..360B. doi:10.1038/d41586-021-00659-y. PMID 33731951. S2CID 232297719.
- ^ "One Russian scientist hopes to slow the thawing of the Arctic". The Economist. 16 December 2020.
- ^ "West Arnhem Land Fire Abatement Project". Savanna Information. Tropical Savannas Cooperative Research Centre. Archived from the original on 3 July 2008. Retrieved 8 July 2008.
- ^ "Eureka Win for West Arnhem Land Fire Project". Savanna Information. Tropical Savannas Cooperative Research Centre. Archived from the original on 3 July 2008. Retrieved 8 July 2008.
- ^ Ning Zeng (2008). "Carbon sequestration via wood burial". Carbon Balance and Management. 3: 1. doi:10.1186/1750-0680-3-1. PMC 2266747. PMID 18173850.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Climate Change, Global Warming, and the Built Environment – Architecture 2030". Retrieved 23 February 2007.
- ^ "Processing Natural Gas". NaturalGas.org. Retrieved 9 February 2018.
- ^ Churkina, Galina; Organschi, Alan; Reyer, Christopher P. O.; Ruff, Andrew; Vinke, Kira; Liu, Zhu; Reck, Barbara K.; Graedel, T. E.; Schellnhuber, Hans Joachim (April 2020). "Buildings as a global carbon sink". Nature Sustainability. 3 (4): 269–276. doi:10.1038/s41893-019-0462-4. S2CID 213032074. Retrieved 20 June 2020.
- ^ European Commission. Directorate General for Research and Innovation; European Commission's Group of Chief Scientific Advisors (2018). Novel carbon capture and utilisation technologies. Publications Office. doi:10.2777/01532. ISBN 978-92-79-82006-9.[page needed]
- ^ a b Erans, María; Sanz-Pérez, Eloy S.; Hanak, Dawid P.; Clulow, Zeynep; Reiner, David M.; Mutch, Greg A. (2022). "Direct air capture: process technology, techno-economic and socio-political challenges". Energy & Environmental Science. 15 (4): 1360–1405. doi:10.1039/D1EE03523A. hdl:10115/19074. S2CID 247178548.
- ^ Keith, David W.; Holmes, Geoffrey; St. Angelo, David; Heide, Kenton (7 June 2018). "A Process for Capturing CO2 from the Atmosphere". Joule. 2 (8): 1573–1594. doi:10.1016/j.joule.2018.05.006.
- ^ Beuttler, Christoph; Charles, Louise; Wurzbacher, Jan (21 November 2019). "The Role of Direct Air Capture in Mitigation of Anthropogenic Greenhouse Gas Emissions". Frontiers in Climate. 1: 10. doi:10.3389/fclim.2019.00010.
- ^ Quarton, Christopher J.; Samsatli, Sheila (1 January 2020). "The value of hydrogen and carbon capture, storage and utilisation in decarbonising energy: Insights from integrated value chain optimisation" (PDF). Applied Energy. 257: 113936. Bibcode:2020ApEn..25713936Q. doi:10.1016/j.apenergy.2019.113936. S2CID 208829001.
- ^ "Carbon-dioxide-removal options are multiplying". The Economist. 20 November 2023.
- ^ "The many prices of carbon dioxide". The Economist. 20 November 2023.
- ^ Sanz-Pérez, Eloy S.; Murdock, Christopher R.; Didas, Stephanie A.; Jones, Christopher W. (12 October 2016). "Direct Capture of carbon dioxide from Ambient Air". Chemical Reviews. 116 (19): 11840–11876. doi:10.1021/acs.chemrev.6b00173. PMID 27560307.
- ^ "Direct Air Capture (Technology Factsheet)" (PDF). Geoengineering Monitor. 24 May 2018. Archived (PDF) from the original on 26 August 2019. Retrieved 27 August 2019.
- ^ a b Brewer, Peter G.; Peltzer, Edward T.; Orr, Franklin M. Jr. (7 May 1999). "Direct Experiments on the Ocean Disposal of Fossil Fuel CO2". Science. 284 (5416): 943–945. Bibcode:1999Sci...284..943B. doi:10.1126/science.284.5416.943. PMID 10320370.
- ^ The Christian Science Monitor (28 April 2008). "Potent greenhouse-gas methane has been rising". The Christian Science Monitor.
- ^ Norman Baker; Ben Bradshaw (4 July 2005). "Carbon Sequestration". Retrieved 8 July 2008.
- ^ Zakem, Emily J.; Mahadevan, Amala; Lauderdale, Jonathan M.; Follows, Michael J. (2020). "Stable aerobic and anaerobic coexistence in anoxic marine zones". The ISME Journal. 14 (1): 288–301. doi:10.1038/s41396-019-0523-8. PMC 6908664. PMID 31624350. S2CID 204758450.
- ^ "Sequestration of Supercritical CO2 in Deep Sedimentary Geological Formations". Negative Emissions Technologies and Reliable Sequestration: A Research Agenda (Report). Washington, DC: The National Academies Press. 2019. pp. 319–350. doi:10.17226/25259. ISBN 978-0-309-48452-7.
- ^ "Methane: A crucial opportunity in the climate fight (Environmental Defense Fund)". Retrieved 18 September 2021.
- ^ Stephanie Flude, Juan Alcade (4 March 2020). "Carbon capture and storage has stalled needlessly".
- ^ "CO2 storage in depleted oilfields" (PDF). 2009.
- ^ "Bureau of Economic Geology Receives $38 Million for First Large-Scale U.S. Test Storing Carbon Dioxide Underground". Jackson School of Geosciences, The University of Texas at Austin. 24 October 2007. Archived from the original on 11 June 2010. Retrieved 14 April 2010.
- ^ "Carbon-capture Technology To Help UK Tackle Global Warming". ScienceDaily. 27 July 2007.
- ^ "Mineral carbonation project for NSW". 9 June 2010.
- ^ a b Frost, B. R.; Beard, J. S. (3 April 2007). "On Silica Activity and Serpentinization". Journal of Petrology. 48 (7): 1351–1368. doi:10.1093/petrology/egm021.
- ^ "New materials can selectively capture CO2, scientists say". CBC News. 15 February 2008.
- ^ "Global Carbon Budget 2021" (PDF). Global Carbon Project. 4 November 2021. p. 57. Archived (PDF) from the original on 11 December 2021.
The cumulative contributions to the global carbon budget from 1850. The carbon imbalance represents the gap in our current understanding of sources & sinks. ... Source: Friedlingstein et al 2021; Global Carbon Project 2021
- ^ Earth Institute News, Columbia University, 18 November 2009
- ^ Knorr, W. (2009). "Is the airborne fraction of anthropogenic CO2 emissions increasing?". Geophysical Research Letters. 36 (21): L21710. Bibcode:2009GeoRL..3621710K. doi:10.1029/2009GL040613.
- "Controversial new climate change results". University of Bristol (Press release). 9 November 2009.
- ^ "Brazilian forests found to be transitioning from carbon sinks to carbon sources". phys.org. Retrieved 16 January 2021.
- ^ Maia, Vinícius Andrade; Santos, Alisson Borges Miranda; Aguiar-Campos, Natália de; Souza, Cléber Rodrigo de; Oliveira, Matheus Coutinho Freitas de; Coelho, Polyanne Aparecida; Morel, Jean Daniel; Costa, Lauana Silva da; Farrapo, Camila Laís; Fagundes, Nathalle Cristine Alencar; Paula, Gabriela Gomes Pires de; Santos, Paola Ferreira; Gianasi, Fernanda Moreira; Silva, Wilder Bento da; Oliveira, Fernanda de; Girardelli, Diego Teixeira; Araújo, Felipe de Carvalho; Vilela, Taynara Andrade; Pereira, Rafaella Tavares; Silva, Lidiany Carolina Arantes da; Menino, Gisele Cristina de Oliveira; Garcia, Paulo Oswaldo; Fontes, Marco Aurélio Leite; Santos, Rubens Manoel dos (1 December 2020). "The carbon sink of tropical seasonal forests in southeastern Brazil can be under threat". Science Advances. 6 (51): eabd4548. Bibcode:2020SciA....6.4548M. doi:10.1126/sciadv.abd4548. ISSN 2375-2548. PMID 33355136.
- ^ Kirn, Marda (2016). Climate Literacy: From the "What" of Climate Change to the "So What" of Personal and Social Change. Geological Society of America Annual Meeting in Denver, Colorado, USA (Abstract). Geological Society of America. doi:10.1130/abs/2016am-287348.
- ^ Schleien, Danny (24 September 2020). "We Need To Harness Natural Carbon Sinks To Reverse Climate Change". Climate Conscious. Retrieved 11 November 2021.
