D-dimer
D-dimer (or D dimer) is a dimer that is a fibrin degradation product (or FDP), a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. It is so named because it contains two D fragments of the fibrin protein joined by a cross-link, hence forming a protein dimer.[1]
D-dimer concentration may be determined by a blood test to help diagnose thrombosis.[2] Since its introduction in the 1990s, it has become an important test performed in people with suspected thrombotic disorders, such as venous thromboembolism.[2][3] While a negative result practically rules out thrombosis, a positive result can indicate thrombosis but does not exclude other potential causes.[3] Its main use, therefore, is to exclude thromboembolic disease where the probability is low.[1][2]
D-dimer levels are used as a predictive biomarker for the blood disorder disseminated intravascular coagulation and in the coagulation disorders associated with COVID-19 infection.[1][3] A four-fold increase in the protein is an indicator of poor prognosis in people hospitalized with COVID-19.[1][3][4]
Principles
[edit]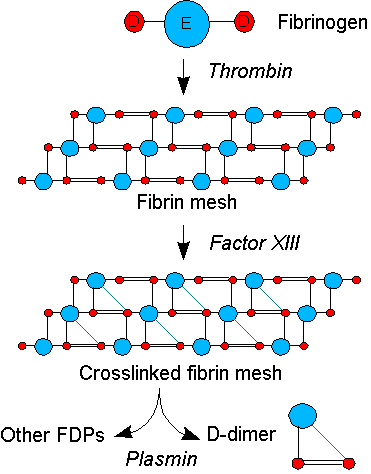
Coagulation, the formation of a blood clot or thrombus, occurs when the proteins of the coagulation cascade are activated, either by contact with a damaged blood vessel wall and exposure to collagen in the tissue space (intrinsic pathway) or by activation of factor VII by tissue activating factors (extrinsic pathway). Both pathways lead to the generation of thrombin, an enzyme that turns the soluble blood protein fibrinogen into fibrin, which aggregates into protofibrils. Another thrombin-generated enzyme, factor XIII, then crosslinks the fibrin protofibrils at the D fragment site, leading to the formation of an insoluble gel that serves as a scaffold for blood clot formation.[1]
The circulating enzyme plasmin, the main enzyme of fibrinolysis, cleaves the fibrin gel in a number of places. The resultant fragments, "high molecular weight polymers", are digested several times more by plasmin to lead to intermediate and then to small polymers (fibrin degradation products or FDPs). The cross-link between two D fragments remains intact, however, and these are exposed on the surface when the fibrin fragments are sufficiently digested. The structure of D-dimer is either a 180 kDa[6] or 195 kDa[7] molecule of two D domains, or a 340 kDa[7] molecule of two D domains and one E domain of the original fibrinogen molecule.[1] The half-life of D-dimer in blood is approximately 6 to 8 hours.[8]
D-dimers are not normally present in human blood plasma, except when the coagulation system has been activated, for instance, because of the presence of thrombosis or disseminated intravascular coagulation. The D-dimer assay depends on the binding of a monoclonal antibody to a particular epitope on the D-dimer fragment. Several detection kits are commercially available; all of them rely on a different monoclonal antibody against D-dimer. For some of these, the area of the D-dimer to which the antibody binds is known. The binding of the antibody is then measured quantitatively by one of various laboratory methods.[1]
Indications
[edit]D-dimer testing is of clinical use when there is a suspicion of deep venous thrombosis (DVTl), pulmonary embolism (PE) or disseminated intravascular coagulation (DIC).[1][3]
For DVT and PE, there are possible various scoring systems that are used to determine the a priori clinical probability of these diseases; the best-known is the Wells score.[5]
- For a high score, or pretest probability, a D-dimer will make little difference and anticoagulant therapy will be initiated regardless of test results, and additional testing for DVT or pulmonary embolism may be performed.
- For a moderate or low score, or pretest probability:[citation needed]
- A negative D-dimer test will virtually rule out thromboembolism:[5] the degree to which the D-dimer reduces the probability of thrombotic disease is dependent on the test properties of the specific test used in the clinical setting: most available D-dimer tests with a negative result will reduce the probability of thromboembolic disease to less than 1% if the pretest probability is less than 15-20%. Chest computed tomography (CT angiography) should not be used to evaluate pulmonary embolism for persons with negative results of a D-dimer assay.[9] A low pretest probability is also valuable in ruling out PE.[10]
- If the D-dimer reads high, then further testing (ultrasound of the leg veins or lung scintigraphy or CT scanning) is required to confirm the presence of thrombus. Anticoagulant therapy may be started at this point or withheld until further tests confirm the diagnosis, depending on the clinical situation.
In some hospitals, they are measured by laboratories after a form is completed showing the probability score and only if the probability score is low or intermediate. This reduces the need for unnecessary tests in those who are high-probability.[11] Performing the D-dimer test first can avoid a significant proportion of imaging tests and is less invasive. Since the D-dimer can exclude the need for imaging, specialty professional organizations recommend that physicians use D-dimer testing as an initial diagnostic.[12][13][14][15]
Interpretation
[edit]Reference ranges
[edit]The following are reference ranges for D-dimer:[16]
| Units | Nonpregnant adult |
First trimester | Second trimester | Third trimester |
|---|---|---|---|---|
| mg/L or μg/mL | < 0.5 | 0.05 - 0.95 | 0.32 - 1.29 | 0.13 -1.7 |
| μg/L or ng/mL | < 500 | 50 - 950 | 320 - 1290 | 130 - 1700 |
| nmol/L | < 2.7 | 0.3 - 5.2 | 1.8 - 7.1 | 0.7 - 9.3 |
D-dimer increases with age. It has therefore been suggested to use a cutoff equal to patient’s age in years × 10 μg/L (or x 0.056 nmol/L) for patients aged over 50 years for the suspicion of venous thromboembolism (VTE), as it decreases the false positive rate without substantially increasing the false negative rate.[17][18]
An alternative measurement of D-dimer is in fibrinogen equivalent units (FEU). The molecular weight of the fibrinogen molecule is about twice the size of the D-dimer molecule, and therefore 1.0 mcg/mL FEU is equivalent to 0.5 mcg/mL of d-dimer.[19]
Thrombotic disease
[edit]Various kits have a 93 to 95% sensitivity (true positive rate). For hospitalized patients, one study found the specificity to be about 50% (related to false positive rate) in the diagnosis of thrombotic disease.[20]
- False positive readings can be due to various causes: liver disease, high rheumatoid factor, inflammation, malignancy, trauma, pregnancy, recent surgery as well as advanced age.[21]
- False negative readings can occur if the sample is taken either too early after thrombus formation or if testing is delayed for several days. Additionally, the presence of anti-coagulation can render the test negative because it prevents thrombus extension. The anti-coagulation medications dabigatran and rivaroxaban decrease D-dimer levels but do not interfere with the D-dimer assay.[22]
- False values may be obtained if the specimen collection tube is not sufficiently filled (false low value if underfilled and false high value if overfilled). This is due to the dilutional effect of the anticoagulant (the blood must be collected in a 9:1 blood to anticoagulant ratio).
- Likelihood ratios are derived from sensitivity and specificity to adjust pretest probability.
In interpretation of the D-dimer, for patients over age 50, a value of (patient's age) × 10 μg/L may be abnormal.[23][24]
History
[edit]D-dimer was originally identified, described and named in the 1970s (Fibrinolysis, Dr P J Gaffney) and found its diagnostic application in the 1990s.[1][5]
References
[edit]- ^ a b c d e f g h i j Asakura, Hidesaku; Ogawa, Haruhiko (2020). "COVID-19-associated coagulopathy and disseminated intravascular coagulation". International Journal of Hematology. 113 (1): 45–57. doi:10.1007/s12185-020-03029-y. ISSN 0925-5710. PMC 7648664. PMID 33161508.
- ^ a b c Khan, Faizan; Tritschler, Tobias; Kahn, Susan R; Rodger, Marc A (2021). "Venous thromboembolism". The Lancet. 398 (10294): 64–77. doi:10.1016/s0140-6736(20)32658-1. ISSN 0140-6736. PMID 33984268. S2CID 234497047.
- ^ a b c d e Ponti, G; Maccaferri, M; Ruini, C; Tomasi, A; Ozben, T (2020). "Biomarkers associated with COVID-19 disease progression". Critical Reviews in Clinical Laboratory Sciences. 57 (6): 389–399. doi:10.1080/10408363.2020.1770685. ISSN 1040-8363. PMC 7284147. PMID 32503382.
- ^ Velavan, Thirumalaisamy P.; Meyer, Christian G. (25 April 2020). "Mild versus severe COVID-19: laboratory markers". International Journal of Infectious Diseases. 95: 304–307. doi:10.1016/j.ijid.2020.04.061. PMC 7194601. PMID 32344011. Retrieved 25 April 2020.
- ^ a b c d Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. (September 2003). "Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis". The New England Journal of Medicine. 349 (13): 1227–35. doi:10.1056/NEJMoa023153. PMID 14507948.
- ^ Kogan AE, Mukharyamova KS, Bereznikova AV, Filatov VL, Koshkina EV, Bloshchitsyna MN, Katrukha AG (July 2016). "Monoclonal antibodies with equal specificity to D-dimer and high-molecular-weight fibrin degradation products". Blood Coagulation & Fibrinolysis. 27 (5): 542–50. doi:10.1097/MBC.0000000000000453. PMC 4935535. PMID 26656897.
- ^ a b Olson JD, Cunningham MT, Higgins RA, Eby CS, Brandt JT (August 2013). "D-dimer: simple test, tough problems". Archives of Pathology & Laboratory Medicine. 137 (8): 1030–8. doi:10.5858/arpa.2012-0296-CP. PMID 23899057.
- ^ Lippi G, Cervellin G, Franchini M, Favaloro EJ (November 2010). "Biochemical markers for the diagnosis of venous thromboembolism: the past, present and future". J Thromb Thrombolysis. 30 (4): 459–71. doi:10.1007/s11239-010-0460-x. PMID 20213258. S2CID 23806848.
- ^ American College of Chest Physicians; American Thoracic Society (September 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American College of Chest Physicians and American Thoracic Society, retrieved 6 January 2013.
- ^ Crawford F, Andras A, Welch K, Sheares K, Keeling D, Chappell FM (August 2016). Cochrane Vascular Group (ed.). "D-dimer test for excluding the diagnosis of pulmonary embolism". The Cochrane Database of Systematic Reviews. 2016 (8): CD010864. doi:10.1002/14651858.CD010864.pub2. PMC 6457638. PMID 27494075.
- ^ Rathbun SW, Whitsett TL, Vesely SK, Raskob GE (March 2004). "Clinical utility of D-dimer in patients with suspected pulmonary embolism and nondiagnostic lung scans or negative CT findings". Chest. 125 (3): 851–5. doi:10.1378/chest.125.3.851. PMC 1215466. PMID 15006941.
- ^ American College of Physicians, "Five Things Physicians and Patients Should Question" (PDF), Choosing Wisely, presented by ABIM Foundation, American College of Physicians, archived from the original (PDF) on June 24, 2012, retrieved August 14, 2012
- ^ Fesmire FM, Brown MD, Espinosa JA, Shih RD, Silvers SM, Wolf SJ, Decker WW (June 2011). "Critical issues in the evaluation and management of adult patients presenting to the emergency department with suspected pulmonary embolism". Annals of Emergency Medicine. 57 (6): 628–652.e75. doi:10.1016/j.annemergmed.2011.01.020. PMID 21621092.
- ^ Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. (September 2008). "Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)". European Heart Journal. 29 (18): 2276–315. doi:10.1093/eurheartj/ehn310. PMID 18757870.
- ^ Qaseem A, Snow V, Barry P, Hornbake ER, Rodnick JE, Tobolic T, et al. (2007). "Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians". Annals of Family Medicine. 5 (1): 57–62. doi:10.1370/afm.667. PMC 1783928. PMID 17261865.
- ^ Reference Values During Pregnancy at perinatology.com. Retrieved October 2014.
- ^ Urban K, Kirley K, Stevermer JJ (March 2014). "PURLs: It's time to use an age-based approach to D-dimer". The Journal of Family Practice. 63 (3): 155–8. PMC 4042909. PMID 24701602.
- ^ Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD (November 2015). "Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians". Annals of Internal Medicine. 163 (9): 701–11. doi:10.7326/M14-1772. PMID 26414967.
- ^ "Clinical Education Center". Quest Diagnostics. Document FAQS.149 Version: 3 effective 07/23/2019 to present
- ^ Schrecengost JE, LeGallo RD, Boyd JC, Moons KG, Gonias SL, Rose CE, Bruns DE (September 2003). "Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism". Clinical Chemistry. 49 (9): 1483–90. doi:10.1373/49.9.1483. PMID 12928229.
- ^ Kabrhel C, Mark Courtney D, Camargo CA, Plewa MC, Nordenholz KE, Moore CL, et al. (June 2010). "Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism". Academic Emergency Medicine. 17 (6): 589–97. doi:10.1111/j.1553-2712.2010.00765.x. PMC 3538031. PMID 20624138.
- ^ Baglin T, Keeling D, Kitchen S (November 2012). "Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology". British Journal of Haematology. 159 (4): 427–9. doi:10.1111/bjh.12052. PMID 22970737.
- ^ van Es J, Mos I, Douma R, Erkens P, Durian M, Nizet T, et al. (January 2012). "The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded". Thrombosis and Haemostasis. 107 (1): 167–71. doi:10.1160/TH11-08-0587. PMID 22072293. S2CID 4832019.
- ^ Douma RA, le Gal G, Söhne M, Righini M, Kamphuisen PW, Perrier A, et al. (March 2010). "Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts". BMJ. 340: c1475. doi:10.1136/bmj.c1475. PMC 2847688. PMID 20354012.
External links
[edit]- D-dimer - Lab Tests Online
