Decane
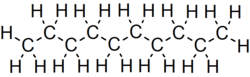
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Decane[1] | |
| Other names
Decyl hydride
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1696981 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.262 |
| EC Number |
|
| MeSH | decane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2247 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H22 | |
| Molar mass | 142.286 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like (in high concentrations) |
| Density | 0.730 g mL−1 |
| Melting point | −30.5 to −29.2 °C; −22.8 to −20.6 °F; 242.7 to 243.9 K |
| Boiling point | 173.8 to 174.4 °C; 344.7 to 345.8 °F; 446.9 to 447.5 K |
| log P | 5.802 |
| Vapor pressure | 195 Pa[2] |
Henry's law
constant (kH) |
2.1 nmol Pa−1 kg−1 |
| -119.74·10−6 cm3/mol | |
| Thermal conductivity | 0.1381 W m−1 K−1 (300 K)[3] |
Refractive index (nD)
|
1.411–1.412 |
| Viscosity |
|
| Thermochemistry | |
Heat capacity (C)
|
315.46 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
425.89 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−302.1–−299.9 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−6779.21–−6777.45 kJ mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable, moderately toxic |
| GHS labelling: | |
 
| |
| Danger | |
| H226, H302, H304, H305 | |
| P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | 46.0 °C (114.8 °F; 319.1 K) |
| 210.0 °C (410.0 °F; 483.1 K) | |
| Explosive limits | 0.8–2.6% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
| Safety data sheet (SDS) | hazard.com |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Decane is an alkane hydrocarbon with the chemical formula C10H22. Although 75 structural isomers are possible for decane, the term usually refers to the normal-decane ("n-decane"), with the formula CH3(CH2)8CH3. All isomers, however, exhibit similar properties and little attention is paid to the composition.[5] These isomers are flammable liquids. Decane is present in small quantities (less than 1%) in gasoline (petrol) and kerosene.[6][7] Like other alkanes, it is a nonpolar solvent, and does not dissolve in water, and is readily combustible. Although it is a component of fuels, it is of little importance as a chemical feedstock, unlike a handful of other alkanes.[8]
Reactions[edit]
Decane undergoes combustion, just like other alkanes. In the presence of sufficient oxygen, it burns to form water and carbon dioxide.
- 2 C10H22 + 31 O2 → 20 CO2 + 22 H2O
With insufficient oxygen, carbon monoxide is also formed.
Physical properties[edit]
It has a surface tension of 0.0238 N·m−1.[9]
See also[edit]
References[edit]
- ^ "decane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 5 January 2012.
- ^ Yaws, Carl L. (1999). Chemical Properties Handbook. New York: McGraw-Hill. pp. 159–179. ISBN 0-07-073401-1.
- ^ Touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical properties of matter - the TPRC data series. Volume 3. Thermal conductivity - nonmetallic liquids and gases. Data book. 1970.
- ^ Dymond, J. H.; Oye, H. A. (1994). "Viscosity of Selected Liquid n-Alkanes". Journal of Physical and Chemical Reference Data. 23 (1): 41–53. Bibcode:1994JPCRD..23...41D. doi:10.1063/1.555943. ISSN 0047-2689.
- ^ "75 Isomers of Decane". The Third Millennium Online! (in Latin). Retrieved 26 July 2021.
- ^ "Petroleum - Chemistry Encyclopedia - reaction, water, uses, elements, examples, gas, number, name". www.chemistryexplained.com. Retrieved 2016-01-28.
- ^ "n-Decane (Annotation)". Hazardous Substances Data Bank (HSDB). National Center for Biotechnology Information. Retrieved 7 July 2022.
- ^ Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (15 June 2000), "Hydrocarbons", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, doi:10.1002/14356007.a13_227, ISBN 3527306730
- ^ Website of Krüss Archived 2013-12-01 at the Wayback Machine (8.10.2009)
External links[edit]
 Media related to Decane at Wikimedia Commons
Media related to Decane at Wikimedia Commons- Material Safety Data Sheet for Decane Archived 23 January 2011 at the Wayback Machine
- CHEMINFO Decane Archived 5 November 2006 at the Wayback Machine

