Unbibium
| Theoretical element | ||||||
|---|---|---|---|---|---|---|
| Unbibium | ||||||
| Pronunciation | /ˌuːnbaɪˈbaɪəm/ | |||||
| Alternative names | element 122, eka-thorium | |||||
| Unbibium in the periodic table | ||||||
| ||||||
| Atomic number (Z) | 122 | |||||
| Group | g-block groups (no number) | |||||
| Period | period 8 (theoretical, extended table) | |||||
| Block | g-block | |||||
| Electron configuration | predictions vary, see text | |||||
| Physical properties | ||||||
| Phase at STP | unknown | |||||
| Atomic properties | ||||||
| Oxidation states | (+4) (predicted)[1] | |||||
| Ionization energies | ||||||
| Other properties | ||||||
| CAS Number | 54576-73-7 | |||||
| History | ||||||
| Naming | IUPAC systematic element name | |||||
Unbibium /uːnˈbɪbiəm/,[3] also referred to as eka-thorium or element 122, is the temporary name of a currently unknown chemical element in the periodic table that has the temporary symbol Ubb and the atomic number 122.
In 2008, it was claimed to have been discovered in natural thorium samples[4] but that claim has now been dismissed by recent repetitions of the experiment using more accurate techniques.
History
Neutron evaporation
The first attempt to synthesize unbibium was performed in 1972 by Flerov et al. at JINR, using the hot fusion reaction:[3]
No atoms were detected and a yield limit of 5 mb (5,000,000,000 pb) was measured. Current results (see flerovium) have shown that the sensitivity of this experiment was too low by at least 6 orders of magnitude.[citation needed]
In 2000, the Gesellschaft für Schwerionenforschung (GSI) performed a very similar experiment with much higher sensitivity:[3]
These results indicate that the synthesis of such heavier elements remains a significant challenge and further improvements of beam intensity and experimental efficiency is required. The sensitivity should be increased to 1 fb.[citation needed]
Another unsuccessful attempt to synthesize unbibium was carried out in 1978 at the GSI, where a natural erbium target was bombarded with xenon-136 ions:[3]
The two attempts in the 1970s to synthesize unbibium were caused by research investigating whether superheavy elements could potentially be naturally occurring.[3]
Compound nucleus fission
Several experiments have been performed between 2000-2004 at the Flerov laboratory of Nuclear Reactions studying the fission characteristics of the compound nucleus 306Ubb. Two nuclear reactions have been used, namely 248Cm + 58Fe and 242Pu + 64Ni.[3] The results have revealed how nuclei such as this fission predominantly by expelling closed shell nuclei such as 132Sn (Z=50, N=82). It was also found that the yield for the fusion-fission pathway was similar between 48Ca and 58Fe projectiles, indicating a possible future use of 58Fe projectiles in superheavy element formation.[5]
Claimed discovery as a naturally occurring element
On April 24, 2008, a group led by Amnon Marinov at the Hebrew University of Jerusalem claimed to have found single atoms of unbibium-292 in naturally occurring thorium deposits at an abundance of between 10−11 and 10−12, relative to thorium.[4] The claim of Marinov et al. was criticized by a part of the scientific community, and Marinov says he has submitted the article to the journals Nature and Nature Physics but both turned it down without sending it for peer review.[6] The unbibium-292 atoms were claimed to be superdeformed or hyperdeformed isomers, with a half-life of at least 100 million years.[3]
A criticism of the technique, previously used in purportedly identifying lighter thorium isotopes by mass spectrometry,[7] was published in Physical Review C in 2008.[8] A rebuttal by the Marinov group was published in Physical Review C after the published comment.[9]
A repeat of the thorium-experiment using the superior method of Accelerator Mass Spectrometry (AMS) failed to confirm the results, despite a 100-fold better sensitivity.[10] This result throws considerable doubt on the results of the Marinov collaboration with regards to their claims of long-lived isotopes of thorium,[7] roentgenium[11] and unbibium.[4] It is still possible that traces of unbibium might only exist in some thorium samples, although this is unlikely.[3]
Naming
Using Mendeleev's nomenclature for unnamed and undiscovered elements, unbihexium should be known as eka-thorium or dvi-cerium. In 1979 IUPAC published recommendations according to which the element was to be called unbibium (with the corresponding symbol of Ubb),[12] a systematic element name as a placeholder, until the discovery of the element is confirmed and a name is decided on. The recommendations are largely ignored among scientists, who call it "element 122", with the symbol of (122) or even simply 122.[13]
Predicted properties
Nuclear stability and isotopes
This section needs expansion. You can help by adding to it. (September 2012) |
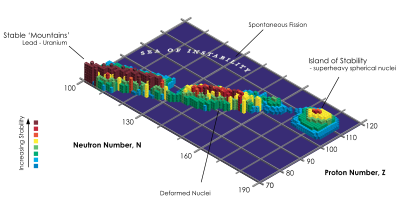
The stability of nuclei decreases greatly with the increase in atomic number after plutonium, the heaviest primordial element, so that all isotopes with an atomic number above 101 decay radioactively with a half-life under a day, with an exception of dubnium-268. No elements with atomic numbers above 82 (after lead) have stable isotopes.[14] Nevertheless, because of reasons not very well understood yet, there is a slight increased nuclear stability around atomic numbers 110–114, which leads to the appearance of what is known in nuclear physics as the "island of stability". This concept, proposed by University of California professor Glenn Seaborg, explains why superheavy elements last longer than predicted.[15] In this region of the periodic table, N=184 and N=196 have been suggested as closed neutron shells. Therefore the isotopes of most interest are 306Ubb and 318Ubb, for these might be considerably longer-lived than other isotopes. Element 122 is predicted to lie within the island of stability, with element 126 predicted to lie near its peak.[3]
Atomic and physical
This section needs expansion. You can help by adding to it. (September 2012) |
Unbibium is predicted to belong to a new block of valence g-electron atoms, although the g-block's position left of the f-block is speculative.[16] It has been predicted by Ephraim Eliav et al. that unbibium will have the electron configuration [Uuo]8s27d18p1,[17] although there may be a smearing out of the energies of 5g, 6f, 7d and 8p orbitals.[16]
Chemical
If group reactivity is followed, unbibium should be a reactive metal, more reactive than cerium or thorium. Unbibium would most likely form the dioxide, UbbO2, and trihalides, such as UbbF3 and UbbCl3. The predicted oxidation states are III and IV (and perhaps II).[citation needed] Being a congener of thorium, it should have similar chemistry to thorium, with multiple oxidation states.[3]
See also
References
- ^ Pyykkö, Pekka (2011). "A suggested periodic table up to Z ≤ 172, based on Dirac–Fock calculations on atoms and ions". Physical Chemistry Chemical Physics. 13 (1): 161–8. Bibcode:2011PCCP...13..161P. doi:10.1039/c0cp01575j. PMID 20967377.
- ^ a b Eliav, E.; Fritzsche, S.; Kaldor, U. (2015). "Electronic structure theory of the superheavy elements". Nuclear Physics A. 944 (December 2015): 518–550. doi:10.1016/j.nuclphysa.2015.06.017.
- ^ a b c d e f g h i j k Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. p. 588. ISBN 978-0-19-960563-7.
- ^ a b c Marinov, A. (2008). "Evidence for a long-lived superheavy nucleus with atomic mass number A=292 and atomic number Z=~122 in natural Th". International Journal of Modern Physics E. 19: 131. arXiv:0804.3869. Bibcode:2010IJMPE..19..131M. doi:10.1142/S0218301310014662.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ see Flerov lab annual reports 2000–2004 inclusive http://www1.jinr.ru/Reports/Reports_eng_arh.html
- ^ Royal Society of Chemistry, "Heaviest element claim criticised", Chemical World.
- ^ a b Marinov, A.; Rodushkin, I.; Kashiv, Y.; Halicz, L.; Segal, I.; Pape, A.; Gentry, R. V.; Miller, H. W.; Kolb, D.; Brandt, R. (2007). "Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes". Phys. Rev. C. 76 (2): 021303(R). arXiv:nucl-ex/0605008. Bibcode:2007PhRvC..76b1303M. doi:10.1103/PhysRevC.76.021303.
- ^ R. C. Barber; J. R. De Laeter (2009). "Comment on "Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes"". Phys. Rev. C. 79 (4): 049801. Bibcode:2009PhRvC..79d9801B. doi:10.1103/PhysRevC.79.049801.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ A. Marinov; I. Rodushkin; Y. Kashiv; L. Halicz; I. Segal; A. Pape; R. V. Gentry; H. W. Miller; D. Kolb; R. Brandt (2009). "Reply to "Comment on 'Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes'"". Phys. Rev. C. 79 (4): 049802. Bibcode:2009PhRvC..79d9802M. doi:10.1103/PhysRevC.79.049802.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J. Lachner; I. Dillmann; T. Faestermann; G. Korschinek; M. Poutivtsev; G. Rugel (2008). "Search for long-lived isomeric states in neutron-deficient thorium isotopes". Phys. Rev. C. 78 (6): 064313. arXiv:0907.0126. Bibcode:2008PhRvC..78f4313L. doi:10.1103/PhysRevC.78.064313.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marinov, A.; Rodushkin, I.; Pape, A.; Kashiv, Y.; Kolb, D.; Brandt, R.; Gentry, R. V.; Miller, H. W.; Halicz, L.; Segal, I. (2009). "Existence of Long-Lived Isotopes of a Superheavy Element in Natural Au" (PDF). International Journal of Modern Physics E. 18 (3). World Scientific Publishing Company: 621–629. arXiv:nucl-ex/0702051. Bibcode:2009IJMPE..18..621M. doi:10.1142/S021830130901280X. Retrieved February 12, 2012.
- ^ Chatt, J. (1979). "Recommendations for the Naming of Elements of Atomic Numbers Greater than 100". Pure Appl. Chem. 51 (2): 381–384. doi:10.1351/pac197951020381.
- ^ Haire, Richard G. (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. p. 1724. ISBN 1-4020-3555-1.
{{cite book}}: CS1 maint: ref duplicates default (link) - ^ Marcillac, Pierre de (April 2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Considine, Glenn D.; Kulik, Peter H. (2002). Van Nostrand's scientific encyclopedia (9 ed.). Wiley-Interscience. ISBN 978-0-471-33230-5. OCLC 223349096.
- ^ a b Seaborg (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. Retrieved 2010-03-16.
- ^ Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. p. 1659. ISBN 1-4020-3555-1.



