Genetic code: Difference between revisions
m →RNA codon table: note incoming redirects |
Undid revision 376549238 by Anthony Appleyard (talk codons are not polar etc but amino acids are) |
||
| Line 35: | Line 35: | ||
|} |
|} |
||
{| class="wikitable" |
{| class="wikitable" |
||
|+ |
|+ |
||
The table shows the 64 codons and the amino acid for each. |
The table shows the 64 codons and the amino acid for each. <br /> |
||
The [[Directionality (molecular biology)|direction]] of the mRNA is 5' to 3'. |
The [[Directionality (molecular biology)|direction]] of the mRNA is 5' to 3'. |
||
|- |
|- |
||
| rowspan=2 colspan=2 | |
| rowspan="2" colspan="2" | |
||
! colspan=4 border=0 | 2nd base |
! colspan="4" border="0" | 2nd base |
||
|- |
|- |
||
! U |
! U |
||
| Line 48: | Line 48: | ||
! G |
! G |
||
|- |
|- |
||
! rowspan=" |
! rowspan="4" border="0" | 1st<br />base |
||
! U |
|||
! rowspan=3 | U |
|||
| |
|||
| style="background:#ffe75f;"| UUU (Phe/F) [[Phenylalanine]]<br /> |
|||
{|style="width:100%;" |
|||
UUC (Phe/F) Phenylalanine |
|||
| style="background:# |
| UUU || style="background:#ffe75f;" |(Phe/F)<br /> [[Phenylalanine]] |
||
UCC (Ser/S) Serine |
|||
| style="background:#b3dec0;"| UAU (Tyr/Y) [[Tyrosine]]<br /> |
|||
UAC (Tyr/Y) Tyrosine |
|||
| style="background:#b3dec0;"| UGU (Cys/C) [[Cysteine]]<br /> |
|||
UGC (Cys/C) Cysteine<br /> |
|||
|- |
|- |
||
| style="background:#ffe75f;"| |
|UUC || style="background:#ffe75f;" | (Phe/F)<br /> [[Phenylalanine]] |
||
| style="background:#b3dec0;"| UCA (Ser/S) Serine |
|||
| style="background:#B0B0B0;"| UAA Ochre (''Stop'') |
|||
| style="background:#B0B0B0;"| UGA Opal (''Stop'') |
|||
|- |
|- |
||
| style="background:#ffe75f;"| |
| UUA || style="background:#ffe75f;" | (Leu/L)<br /> [[Leucine]] |
||
| style="background:#b3dec0;"| UCG (Ser/S) Serine |
|||
| style="background:#B0B0B0;"| UAG Amber (''Stop'') |
|||
| style="background:#ffe75f;"| UGG (Trp/W) [[Tryptophan]] |
|||
|- |
|||
!rowspan=2| C |
|||
| style="background:#ffe75f;"| CUU (Leu/L) Leucine<br /> |
|||
CUC (Leu/L) Leucine |
|||
| style="background:#ffe75f;"| CCU (Pro/P) [[Proline]]<br /> |
|||
CCC (Pro/P) Proline |
|||
| style="background:#bbbfe0;"| CAU (His/H) [[Histidine]]<br /> |
|||
CAC (His/H) Histidine |
|||
| style="background:#bbbfe0;"| CGU (Arg/R) [[Arginine]]<br /> |
|||
CGC (Arg/R) Arginine |
|||
|- |
|- |
||
| style="background:#ffe75f;" |
| UUG || style="background:#ffe75f;" | (Leu/L)<br /> [[Leucine]] |
||
|} |
|||
CUG (Leu/L) Leucine<br /> |
|||
| |
|||
| style="background:#ffe75f;"| CCA (Pro/P) Proline<br /> |
|||
{| style="width:100%;" |
|||
CCG (Pro/P) Proline<br /> |
|||
| style="background:#b3dec0;"| |
| UCU || style="background:#b3dec0;" | (Ser/S)<br /> [[Serine]] |
||
CAG (Gln/Q) Glutamine<br /> |
|||
| style="background:#bbbfe0;"| CGA (Arg/R) Arginine<br /> |
|||
CGG (Arg/R) Arginine |
|||
|- |
|||
! rowspan=3| A |
|||
| style="background:#ffe75f;"| AUU (Ile/I) [[Isoleucine]]<br /> |
|||
AUC (Ile/I) Isoleucine |
|||
| style="background:#b3dec0;"| ACU (Thr/T) [[Threonine]]<br /> |
|||
ACC (Thr/T) Threonine |
|||
| style="background:#b3dec0;"| AAU (Asn/N) [[Asparagine]]<br /> |
|||
AAC (Asn/N) Asparagine |
|||
| style="background:#b3dec0;"| AGU (Ser/S) Serine<br /> |
|||
AGC (Ser/S) Serine |
|||
|- |
|- |
||
| style="background:# |
| UCC || style="background:#b3dec0;" | (Ser/S)<br /> [[Serine]] |
||
| style="background:#b3dec0;"| ACA (Thr/T) Threonine |
|||
| style="background:#bbbfe0;"| AAA (Lys/K) [[Lysine]] |
|||
| style="background:#bbbfe0;"| AGA (Arg/R) Arginine |
|||
|- |
|- |
||
| style="background:# |
| UCA || style="background:#b3dec0;" | (Ser/S)<br /> [[Serine]] |
||
|- |
|||
| style="background:#b3dec0;"| ACG (Thr/T) Threonine |
|||
| style="background:# |
| UCG || style="background:#b3dec0;" | (Ser/S)<br /> [[Serine]] |
||
|} |
|||
| style="background:#bbbfe0;"| AGG (Arg/R) Arginine |
|||
| |
| |
||
{| style="width:100%;" |
|||
!rowspan=3| G |
|||
| style="background:# |
| UAU || style="background:#b3dec0;" | (Tyr/Y)<br /> [[Tyrosine]] |
||
|- |
|||
GUC (Val/V) Valine |
|||
| style="background:# |
| UAC || style="background:#b3dec0;" | (Tyr/Y)<br /> [[Tyrosine]] |
||
|- |
|||
GCC (Ala/A) Alanine |
|||
| style="background:# |
| UAA || style="background:#B0B0B0;"| Ochre <br />''[[Stop_codon|Stop]]'' |
||
|- |
|||
GAC (Asp/D) Aspartic acid |
|||
| style="background:# |
| UAG || style="background:#B0B0B0;"| Amber<br /> ''[[Stop_codon|Stop]]'' |
||
|} |
|||
GGC (Gly/G) Glycine |
|||
| |
|||
{| style="width:100%;" |
|||
| UGU || style="background:#b3dec0;" | (Cys/C)<br /> [[Cysteine]] |
|||
|- |
|||
| UGC || style="background:#b3dec0;" | (Cys/C)<br /> [[Cysteine]] |
|||
|- |
|||
| UGA || style="background:#B0B0B0;"| Opal<br /> ''[[Stop_codon|Stop]]'' |
|||
|- |
|||
| UGG || style="background:#ffe75f;" | (Trp/W)<br /> [[Tryptophan]] |
|||
|} |
|||
|- |
|||
! C |
|||
| |
|||
{| style="width:100%;" |
|||
| CUU || style="background:#ffe75f;" | (Leu/L)<br /> [[Leucine]] |
|||
|- |
|||
| CUC || style="background:#ffe75f;" | (Leu/L)<br /> [[Leucine]] |
|||
|- |
|||
| CUA || style="background:#ffe75f;" | (Leu/L)<br /> [[Leucine]] |
|||
|- |
|||
| CUG || style="background:#ffe75f;" | (Leu/L)<br /> [[Leucine]] |
|||
|} |
|||
| |
|||
{| style="width:100%;" |
|||
| CCU || style="background:#ffe75f;" | (Pro/P)<br /> [[Proline]] |
|||
|- |
|||
| CCC || style="background:#ffe75f;" | (Pro/P)<br /> [[Proline]] |
|||
|- |
|||
| CCA || style="background:#ffe75f;" | (Pro/P)<br /> [[Proline]] |
|||
|- |
|||
| CCG || style="background:#ffe75f;" | (Pro/P)<br /> [[Proline]] |
|||
|} |
|||
| |
|||
{| style="width:100%;" |
|||
| CAU || style="background:#bbbfe0;" | (His/H)<br /> [[Histidine]] |
|||
|- |
|||
| CAC || style="background:#bbbfe0;" | (His/H)<br /> [[Histidine]] |
|||
|- |
|||
| CAA || style="background:#b3dec0;" | (Gln/Q)<br /> [[Glutamine]] |
|||
|- |
|||
| CAG || style="background:#b3dec0;" | (Gln/Q)<br /> [[Glutamine]] |
|||
|} |
|||
| |
|||
{| style="width:100%;" |
|||
| CGU || style="background:#bbbfe0;" | (Arg/R)<br /> [[Arginine]] |
|||
|- |
|||
| CGC || style="background:#bbbfe0;" | (Arg/R)<br /> [[Arginine]] |
|||
|- |
|||
| CGA || style="background:#bbbfe0;" | (Arg/R)<br /> [[Arginine]] |
|||
|- |
|||
| CGG || style="background:#bbbfe0;" | (Arg/R)<br /> [[Arginine]] |
|||
|} |
|||
|- |
|||
! A |
|||
| |
|||
{| style="width:100%;" |
|||
| AUU || style="background:#ffe75f;" | (Ile/I)<br /> [[Isoleucine]] |
|||
|- |
|||
| AUC || style="background:#ffe75f;" | (Ile/I)<br /> [[Isoleucine]] |
|||
|- |
|||
| AUA || style="background:#ffe75f;" | (Ile/I)<br /> [[Isoleucine]] |
|||
|- |
|||
| AUG {{ref label|methionine|A|A}} || style="background:#ffe75f;"| (Met/M)<br /> [[Methionine]] |
|||
|} |
|||
| |
|||
{| style="width:100%;" |
|||
| ACU || style="background:#b3dec0;"| (Thr/T)<br /> [[Threonine]] |
|||
|- |
|||
| ACC || style="background:#b3dec0;"| (Thr/T)<br /> [[Threonine]] |
|||
|- |
|||
| ACA || style="background:#b3dec0;"| (Thr/T)<br /> [[Threonine]] |
|||
|- |
|||
| ACG || style="background:#b3dec0;"| (Thr/T)<br /> [[Threonine]] |
|||
|} |
|||
| |
|||
{| style="width:100%;" |
|||
| AAU || style="background:#b3dec0;"| (Asn/N)<br /> [[Asparagine]] |
|||
|- |
|||
| AAC || style="background:#b3dec0;"| (Asn/N)<br /> [[Asparagine]] |
|||
|- |
|||
| AAA || style="background:#bbbfe0;"| (Lys/K)<br /> [[Lysine]] |
|||
|- |
|||
| AAG || style="background:#bbbfe0;"| (Lys/K)<br /> [[Lysine]] |
|||
|} |
|||
| |
|||
{| style="width:100%;" |
|||
| AGU || style="background:#b3dec0;" | (Ser/S)<br /> [[Serine]] |
|||
|- |
|||
| AGC || style="background:#b3dec0;" | (Ser/S)<br /> [[Serine]] |
|||
|- |
|||
| AGA || style="background:#bbbfe0;" | (Arg/R)<br /> [[Arginine]] |
|||
|- |
|||
| AGG || style="background:#bbbfe0;" | (Arg/R)<br /> [[Arginine]] |
|||
|} |
|||
|- |
|||
! G |
|||
| |
|||
{| style="width:100%;" |
|||
| GUU || style="background:#ffe75f;"| (Val/V)<br /> [[Valine]] |
|||
|- |
|||
| GUC || style="background:#ffe75f;"| (Val/V)<br /> [[Valine]] |
|||
|- |
|||
| GUA || style="background:#ffe75f;"| (Val/V)<br /> [[Valine]] |
|||
|- |
|||
| GUG || style="background:#ffe75f;"| (Val/V)<br /> [[Valine]] |
|||
|} |
|||
| |
|||
{| style="width:100%;" |
|||
| GCU || style="background:#ffe75f;"| (Ala/A)<br /> [[Alanine]] |
|||
|- |
|||
| GCC || style="background:#ffe75f;"| (Ala/A)<br /> [[Alanine]] |
|||
|- |
|||
| GCA || style="background:#ffe75f;"| (Ala/A)<br /> [[Alanine]] |
|||
|- |
|||
| GCG || style="background:#ffe75f;"| (Ala/A)<br /> [[Alanine]] |
|||
|} |
|||
| |
|||
{| style="width:100%;" |
|||
| GAU || style="background:#f8b7d3;"| (Asp/D)<br /> [[Aspartic acid]] |
|||
|- |
|||
| GAC || style="background:#f8b7d3;"| (Asp/D)<br /> [[Aspartic acid]] |
|||
|- |
|||
| GAA || style="background:#f8b7d3;"| (Glu/E)<br /> [[Glutamic acid]] |
|||
|- |
|||
| GAG || style="background:#f8b7d3;"| (Glu/E)<br /> [[Glutamic acid]] |
|||
|} |
|||
| |
|||
{| style="width:100%;" |
|||
| GGU || style="background:#ffe75f;"| (Gly/G)<br /> [[Glycine]] |
|||
|- |
|||
| GGC || style="background:#ffe75f;"| (Gly/G)<br /> [[Glycine]] |
|||
|- |
|||
| GGA || style="background:#ffe75f;"| (Gly/G)<br /> [[Glycine]] |
|||
|- |
|- |
||
| style="background:#ffe75f;"| |
| GGG || style="background:#ffe75f;"| (Gly/G)<br /> [[Glycine]] |
||
|} |
|||
GUG (Val/V) Valine |
|||
| style="background:#ffe75f;"| GCA (Ala/A) Alanine<br /> |
|||
GCG (Ala/A) Alanine |
|||
| style="background:#f8b7d3;"| GAA (Glu/E) [[Glutamic acid]]<br /> |
|||
GAG (Glu/E) Glutamic acid |
|||
| style="background:#ffe75f;"| GGA (Gly/G) Glycine<br /> |
|||
GGG (Gly/G) Glycine |
|||
|} |
|} |
||
Revision as of 00:34, 11 August 2010

The genetic code is the set of rules by which information encoded in genetic material (DNA or mRNA sequences) is translated into proteins (amino acid sequences) by living cells. The code defines a mapping between tri-nucleotide sequences, called codons, and amino acids. With some exceptions,[1] a triplet codon in a nucleic acid sequence specifies a single amino acid. Because the vast majority of genes are encoded with exactly the same code (see the RNA codon table), this particular code is often referred to as the canonical or standard genetic code, or simply the genetic code, though in fact there are many variant codes. For example, protein synthesis in human mitochondria relies on a genetic code that differs from the standard genetic code.
Not all genetic information is stored using the genetic code. All organisms' DNA contains regulatory sequences, intergenic segments, and chromosomal structural areas that can contribute greatly to phenotype. Those elements operate under sets of rules that are distinct from the codon-to-amino acid paradigm underlying the genetic code.
Discovery

After the structure of DNA was deciphered by James Watson, Francis Crick, Maurice Wilkins and Rosalind Franklin, serious efforts to understand the nature of the encoding of proteins began. George Gamow postulated that a three-letter code must be employed to encode the 20 standard amino acids used by living cells to encode proteins, because 3 is the smallest integer n such that 4n is at least 20.[2]
The fact that codons consist of three DNA bases was first demonstrated in the Crick, Brenner et al. experiment. The first elucidation of a codon was done by Marshall Nirenberg and Heinrich J. Matthaei in 1961 at the National Institutes of Health. They used a cell-free system to translate a poly-uracil RNA sequence (i.e., UUUUU...) and discovered that the polypeptide that they had synthesized consisted of only the amino acid phenylalanine. They thereby deduced that the codon UUU specified the amino acid phenylalanine. This was followed by experiments in the laboratory of Severo Ochoa demonstrating that the poly-adenine RNA sequence (AAAAA...) coded for the polypeptide, poly-lysine.[3] and the poly-cytosine RNA sequence (CCCCC...) coded for the polypeptide, poly-proline.[4] Therefore the codon AAA specified the amino acid lysine, and the codon CCC specified the amino acid proline. Using different copolymers most of the remaining codons were then determined. Extending this work, Nirenberg and Philip Leder revealed the triplet nature of the genetic code and allowed the codons of the standard genetic code to be deciphered. In these experiments various combinations of mRNA were passed through a filter which contained ribosomes, the components of cells that translate RNA into protein. Unique triplets promoted the binding of specific tRNAs to the ribosome. Leder and Nirenberg were able to determine the sequences of 54 out of 64 codons in their experiments.[5]
Subsequent work by Har Gobind Khorana identified the rest of the genetic code. Shortly thereafter, Robert W. Holley determined the structure of transfer RNA (tRNA), the adapter molecule that facilitates the process of translating RNA into protein. This work was based upon earlier studies by Severo Ochoa, who received the Nobel prize in 1959 for his work on the enzymology of RNA synthesis.[6] In 1968, Khorana, Holley and Nirenberg received the Nobel Prize in Physiology or Medicine for their work.[7]
Transfer of information via the genetic code
The genome of an organism is inscribed in DNA, or in the case of some viruses, RNA. The portion of the genome that codes for a protein or an RNA is referred to as a gene. Those genes that code for proteins are composed of tri-nucleotide units called codons, each coding for a single amino acid. Each nucleotide sub-unit consists of a phosphate, deoxyribose sugar and one of the 4 nitrogenous nucleobases. The purine bases adenine (A) and guanine (G) are larger and consist of two aromatic rings. The pyrimidine bases cytosine (C) and thymine (T) are smaller and consist of only one aromatic ring. In the double-helix configuration, two strands of DNA are joined to each other by hydrogen bonds in an arrangement known as base pairing. These bonds almost always form between an adenine base on one strand and a thymine on the other strand and between a cytosine base on one strand and a guanine base on the other. This means that the number of A and T residues will be the same in a given double helix, as will the number of G and C residues.[8]: 102–117 In RNA, thymine (T) is replaced by uracil (U), and the deoxyribose is substituted by ribose.[8]: 127
Each protein-coding gene is transcribed into a template molecule of the related polymer RNA, known as messenger RNA or mRNA. This, in turn, is translated on the ribosome into an amino acid chain or polypeptide.[8]: Chp 12 The process of translation requires transfer RNAs specific for individual amino acids with the amino acids covalently attached to them, guanosine triphosphate as an energy source, and a number of translation factors. tRNAs have anticodons complementary to the codons in mRNA and can be "charged" covalently with amino acids at their 3' terminal CCA ends. Individual tRNAs are charged with specific amino acids by enzymes known as aminoacyl tRNA synthetases, which have high specificity for both their cognate amino acids and tRNAs. The high specificity of these enzymes is a major reason why the fidelity of protein translation is maintained.[8]: 464–469
There are 4³ = 64 different codon combinations possible with a triplet codon of three nucleotides; all 64 codons are assigned for either amino acids or stop signals during translation. If, for example, an RNA sequence, UUUAAACCC is considered and the reading frame starts with the first U (by convention, 5' to 3'), there are three codons, namely, UUU, AAA and CCC, each of which specifies one amino acid. This RNA sequence will be translated into an amino acid sequence, three amino acids long.[8]: 521–539 A comparison may be made with computer science, where the codon is similar to a word, which is the standard "chunk" for handling data (like one amino acid of a protein), and a nucleotide is similar to a bit, in that it is the smallest unit.
The standard genetic code is shown in the following tables. Table 1 shows what amino acid each of the 64 codons specifies. Table 2 shows what codons specify each of the 20 standard amino acids involved in translation. These are called forward and reverse codon tables, respectively. For example, the codon AAU represents the amino acid asparagine, and UGU and UGC represent cysteine (standard three-letter designations, Asn and Cys, respectively).[8]: 522
RNA codon table
| nonpolar | polar | basic | acidic | (stop codon) |
| 2nd base | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U | C | A | G | ||||||||||||||||||||||||||||||||||
| 1st base |
U |
|
|
|
| ||||||||||||||||||||||||||||||||
| C |
|
|
|
| |||||||||||||||||||||||||||||||||
| A |
|
|
|
| |||||||||||||||||||||||||||||||||
| G |
|
|
|
| |||||||||||||||||||||||||||||||||
- A The codon AUG both codes for methionine and serves as an initiation site: the first AUG in an mRNA's coding region is where translation into protein begins.[9]
| Ala/A | GCU, GCC, GCA, GCG | Leu/L | UUA, UUG, CUU, CUC, CUA, CUG |
|---|---|---|---|
| Arg/R | CGU, CGC, CGA, CGG, AGA, AGG | Lys/K | AAA, AAG |
| Asn/N | AAU, AAC | Met/M | AUG |
| Asp/D | GAU, GAC | Phe/F | UUU, UUC |
| Cys/C | UGU, UGC | Pro/P | CCU, CCC, CCA, CCG |
| Gln/Q | CAA, CAG | Ser/S | UCU, UCC, UCA, UCG, AGU, AGC |
| Glu/E | GAA, GAG | Thr/T | ACU, ACC, ACA, ACG |
| Gly/G | GGU, GGC, GGA, GGG | Trp/W | UGG |
| His/H | CAU, CAC | Tyr/Y | UAU, UAC |
| Ile/I | AUU, AUC, AUA | Val/V | GUU, GUC, GUA, GUG |
| START | AUG | STOP | UAA, UGA, UAG |
Salient features
Sequence reading frame
A codon is defined by the initial nucleotide from which translation starts. For example, the string GGGAAACCC, if read from the first position, contains the codons GGG, AAA and CCC; and, if read from the second position, it contains the codons GGA and AAC; if read starting from the third position, GAA and ACC. Every sequence can thus be read in three reading frames, each of which will produce a different amino acid sequence (in the given example, Gly-Lys-Pro, Gly-Asn, or Glu-Thr, respectively). With double-stranded DNA there are six possible reading frames, three in the forward orientation on one strand and three reverse on the opposite strand.[10]: 330
The actual frame in which a protein sequence is translated is defined by a start codon, usually the first AUG codon in the mRNA sequence. Mutations that disrupt the reading frame by insertions or deletions of a non-multiple of 3 nucleotide bases are known as frameshift mutations. These mutations may impair the function of the resulting protein, if it is formed, and are thus rare in in vivo protein-coding sequences. Such misformed proteins are often targeted for proteolytic degradation. In addition, a frame shift mutation is very likely to cause a stop codon to be read, which truncates the creation of the protein.[11] One reason for the rareness of frame-shifted mutations' being inherited is that, if the protein being translated is essential for growth under the selective pressures the organism faces, absence of a functional protein may cause death before the organism is viable.[12]
Start/stop codons
Translation starts with a chain initiation codon (start codon). Unlike stop codons, the codon alone is not sufficient to begin the process. Nearby sequences (such as the Shine-Dalgarno sequence in E. coli) and initiation factors are also required to start translation. The most common start codon is AUG which is read as methionine or, in bacteria, as formylmethionine. Alternative start codons (depending on the organism), include "GUG" or "UUG", which normally code for valine or leucine, respectively. However, when used as a start codon, these alternative start codons are translated as methionine or formylmethionine.[13]
The three stop codons have been given names: UAG is amber, UGA is opal (sometimes also called umber), and UAA is ochre. "Amber" was named by discoverers Richard Epstein and Charles Steinberg after their friend Harris Bernstein, whose last name means "amber" in German. The other two stop codons were named "ochre" and "opal" in order to keep the "color names" theme. Stop codons are also called "termination" or "nonsense" codons and they signal release of the nascent polypeptide from the ribosome due to binding of release factors in the absence of cognate tRNAs with anticodons complementary to these stop signals.[14]
Effect of mutations

Frameshift mutations altering the sequence reading frame, and nonsense mutations causing a stop codon are examples of point mutations. In addition, there may be missense mutations that cause exchange of one amino acid for another. Clinically important missense mutations generally change the properties of the coded amino acid residue between being basic, acidic polar or nonpolar, while nonsense mutations result in a stop codon.[10]: 266
Degeneracy of the genetic code
The genetic code has redundancy but no ambiguity (see the codon tables above for the full correlation). For example, although codons GAA and GAG both specify glutamic acid (redundancy), neither of them specifies any other amino acid (no ambiguity). The codons encoding one amino acid may differ in any of their three positions. For example the amino acid glutamic acid is specified by GAA and GAG codons (difference in the third position), the amino acid leucine is specified by UUA, UUG, CUU, CUC, CUA, CUG codons (difference in the first or third position), while the amino acid serine is specified by UCA, UCG, UCC, UCU, AGU, AGC (difference in the first, second or third position).[8]: 521–522
A position of a codon is said to be a fourfold degenerate site if any nucleotide at this position specifies the same amino acid. For example, the third position of the glycine codons (GGA, GGG, GGC, GGU) is a fourfold degenerate site, because all nucleotide substitutions at this site are synonymous; i.e., they do not change the amino acid. Only the third positions of some codons may be fourfold degenerate.[8]: 521–522 A position of a codon is said to be a twofold degenerate site if only two of four possible nucleotides at this position specify the same amino acid. For example, the third position of the glutamic acid codons (GAA, GAG) is a twofold degenerate site. In twofold degenerate sites, the equivalent nucleotides are always either two purines (A/G) or two pyrimidines (C/U), so only transversional substitutions (purine to pyrimidine or pyrimidine to purine) in twofold degenerate sites are nonsynonymous.[8]: 521–522 A position of a codon is said to be a non-degenerate site if any mutation at this position results in amino acid substitution. There is only one threefold degenerate site where changing to three of the four nucleotides may have no effect on the amino acid (depending on what it is changed to), while changing to the fourth possible nucleotide always results in an amino acid substitution. This is the third position of an isoleucine codon: AUU, AUC, or AUA all encode isoleucine, but AUG encodes methionine. In computation this position is often treated as a twofold degenerate site.[8]: 521–522
There are three amino acids encoded by six different codons: serine, leucine, and arginine. Only two amino acids are specified by a single codon. One of these is the amino-acid methionine, specified by the codon AUG, which also specifies the start of translation; the other is tryptophan, specified by the codon UGG. The degeneracy of the genetic code is what accounts for the existence of synonymous mutations.[8]: Chp 15
Degeneracy results because a triplet code designates 20 amino acids and a stop codon. Because there are four bases, triplet codons are required to produce at least 21 different codes. For example, if there were two bases per codon, then only 16 amino acids could be coded for (4²=16). Because at least 21 codes are required, then 4³ gives 64 possible codons, meaning that some degeneracy must exist.[8]: 521–522
These properties of the genetic code make it more fault-tolerant for point mutations. For example, in theory, fourfold degenerate codons can tolerate any point mutation at the third position, although codon usage bias restricts this in practice in many organisms; twofold degenerate codons can tolerate one out of the three possible point mutations at the third position. Since transition mutations (purine to purine or pyrimidine to pyrimidine mutations) are more likely than transversion (purine to pyrimidine or vice-versa) mutations, the equivalence of purines or that of pyrimidines at twofold degenerate sites adds a further fault-tolerance.[8]: 531–532
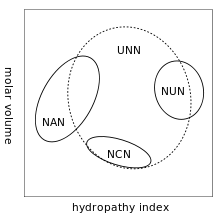
A practical consequence of redundancy is that some errors in the genetic code only cause a silent mutation or an error that would not affect the protein because the hydrophilicity or hydrophobicity is maintained by equivalent substitution of amino acids; for example, a codon of NUN (where N = any nucleotide) tends to code for hydrophobic amino acids. NCN yields amino acid residues that are small in size and moderate in hydropathy; NAN encodes average size hydrophilic residues.[16][17] These tendencies may result from the shared ancestry of the aminoacyl tRNA synthetases related to these codons.
Even so, single point mutations can still cause dysfunctional proteins. For example, a mutated hemoglobin gene causes sickle-cell disease. In the mutant hemoglobin a hydrophilic glutamate (Glu) is substituted by the hydrophobic valine (Val), that is, GAA or GAG becomes GUA or GUG. The substitution of glutamate by valine reduces the solubility of β-globin which causes hemoglobin to form linear polymers linked by the hydrophobic interaction between the valine groups causing sickle-cell deformation of erythrocytes. Sickle-cell disease is generally not caused by a de novo mutation. Rather it is selected for in malarial regions (in a way similar to thalassemia), as heterozygous people have some resistance to the malarial Plasmodium parasite (heterozygote advantage).[18]
These variable codes for amino acids are allowed because of modified bases in the first base of the anticodon of the tRNA, and the base-pair formed is called a wobble base pair. The modified bases include inosine and the Non-Watson-Crick U-G basepair.[19]
Variations to the standard genetic code
While slight variations on the standard code had been predicted earlier,[20] none were discovered until 1979, when researchers studying human mitochondrial genes discovered they used an alternative code. Many slight variants have been discovered since,[21] including various alternative mitochondrial codes,[22] as well as small variants such as Mycoplasma translating the codon UGA as tryptophan and Candida species translating CUG as a serine rather than a leucine.[23][24] In bacteria and archaea, GUG and UUG are common start codons. However, in rare cases, certain specific proteins may use alternative initiation (start) codons not normally used by that species.[21]
In certain proteins, non-standard amino acids are substituted for standard stop codons, depending upon associated signal sequences in the messenger RNA: UGA can code for selenocysteine and UAG can code for pyrrolysine as discussed in the relevant articles. Selenocysteine is now viewed as the 21st amino acid, and pyrrolysine is viewed as the 22nd.[21]
Notwithstanding these differences, all known codes have strong similarities to each other, and the coding mechanism is the same for all organisms: three-base codons, tRNA, ribosomes, reading the code in the same direction and translating the code three letters at a time into sequences of amino acids.
Expanded genetic code
Since 2001, 40 non-natural amino acids have been added into protein by creating a unique codon (recoding) and a corresponding transfer-RNA:aminoacyl – tRNA-synthetase pair to encode it with diverse physicochemical and biological properties in order to be used as a tool to exploring protein structure and function or to create novel or enhanced proteins.[25][26]
Theories on the origin of the genetic code
Despite the minor variations that exist, the genetic code used by all known forms of life is nearly universal. However, there are a huge number of possible genetic codes. If amino acids are randomly associated with triplet codons, there will be 1.5 x 1084 possible genetic codes.[27] The question arises: why this code? How did it originate?
Phylogenetic analysis of transfer RNA suggests that tRNA molecules evolved before the present set of aminoacyl-tRNA synthetases.[28]
Theoretically the genetic code could be completely random (a "frozen accident"), completely non-random (optimal) or a combination of random and nonrandom. There are sufficient data to refute the first possibility.[29] For a start, a quick view on the table of the genetic code already shows a clustering of amino acid assignments. Furthermore, amino acids that share the same biosynthetic pathway tend to have the same first base in their codons,[30] and amino acids with similar physical properties tend to have similar codons.[31][32]
There are four themes running through the many theories that seek to explain the evolution of the genetic code (and hence the origin of these patterns):[33]
- Chemical principles govern specific RNA interaction with amino acids. Aptamer experiments showed that some amino acids have a selective chemical affinity for the base triplets that code for them[34]. Recent experiments show that of the 8 amino acids tested, 6 show some RNA triplet-amino acid association[35] [36]. This has been called the stereochemical code. The stereochemical code could have created an ancient core of assignments. The current complex translation mechanism involving tRNA and associated enzymes may be a later development, and that originally, protein sequences were directly templated on base sequences.
- Biosynthetic expansion. The standard modern genetic code grew from a simpler earlier code through a process of "biosynthetic expansion". Here the idea is that primordial life "discovered" new amino acids (e.g., as by-products of metabolism) and later back-incorporated some of these into the machinery of genetic coding. Although much circumstantial evidence has been found to suggest that fewer different amino acids were used in the past than today,[37] precise and detailed hypotheses about exactly which amino acids entered the code in exactly what order have proved far more controversial.[38][39]
- Natural selection has led to codon assignments of the genetic code that minimize the effects of mutations.[40] A recent hypothesis[41] suggests that the triplet code was derived from codes that used longer than triplet codons. Longer than triplet decoding has higher degree of codon redundancy and is more error resistant than the triplet decoding. This feature could allow accurate decoding in the absence of highly complex translational machinery such as the ribosome.
- The biocommunicative approach investigates nucleotide sequences as code, i.e. language-like text, which follows in parallel three (3) kinds of rules: combinatorial (syntactic), context-sensitive (pragmatic) and content-specific (semantic). Natural genome editing from this perspective is competent viral-driven generation and integration of meaningful nucleotide sequences into pre-existing genomic content arrangements and the ability to (re-)combine and (re-)regulate them according to context-dependent (i.e. adaptational) purposes of the host organism.[42]
- Information-theoretic approaches treat the genetic code in terms of an error-prone information channel. The inherent noise in the channel poses the organism with a fundamental question: how to construct a genetic code that can withstand the impact of noise while accurately and efficiently translating information? These “rate-distortion” models suggest that the genetic code originated as a result of the interplay of the three conflicting evolutionary forces: the needs for diverse amino-acids, for error-tolerance and for minimal cost of resources. The code emerges at a coding phase transition when the mapping of codons to amino-acids becomes nonrandom. The emergence of the code is governed by the topology defined by the most probable errors and is related to the map coloring problem [43].
References
- ^ Turanov AA, Lobanov AV, Fomenko DE, Morrison HG, Sogin ML, Klobutcher LA, Hatfield DL, Gladyshev VN (2009). "Genetic code supports targeted insertion of two amino acids by one codon". Science. 323 (5911): 259–61. doi:10.1126/science.1164748. PMID 19131629.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Crick, Francis (1988). "Chapter 8: The genetic code". What mad pursuit: a personal view of scientific discovery. New York: Basic Books. pp. 89–101. ISBN 0-465-09138-5.
- ^ Gardner RS, Wahba AJ, Basilio C, Miller RS, Lengyel P, Speyer JF (1962). "Synthetic polynucleotides and the amino acid code. VII". Proc. Natl. Acad. Sci. U.S.A. 48: 2087–94. doi:10.1073/pnas.48.12.2087. PMC 221128. PMID 13946552.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wahba AJ, Gardner RS, Basilio C, Miller RS, Speyer JF, Lengyel P (1963). "Synthetic polynucleotides and the amino acid code. VIII". Proc. Natl. Acad. Sci. U.S.A. 49: 116–22. doi:10.1073/pnas.49.1.116. PMC 300638. PMID 13998282.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nirenberg M, Leder P, Bernfield M, Brimacombe R, Trupin J, Rottman F, O'Neal C (1965). "RNA codewords and protein synthesis, VII. On the general nature of the RNA code". Proc. Natl. Acad. Sci. U.S.A. 53 (5): 1161–8. doi:10.1073/pnas.53.5.1161. PMC 301388. PMID 5330357.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "The Nobel Prize in Physiology or Medicine 1959" (Press release). The Royal Swedish Academy of Science. 1959. Retrieved 2010-02-27.
- ^ "The Nobel Prize in Physiology or Medicine 1968" (Press release). The Royal Swedish Academy of Science. 1968. Retrieved 2010-02-27.
- ^ a b c d e f g h i j k l m Watson JD, Baker TA, Bell SP, Gann A, Levine M, Oosick R. (2008). Molecular Biology of the Gene. San Francisco: Pearson/Benjamin Cummings. ISBN 0-8053-9592-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Nakamoto T (2009). "Evolution and the universality of the mechanism of initiation of protein synthesis". Gene. 432 (1–2): 1–6. doi:10.1016/j.gene.2008.11.001. PMID 19056476.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Pamela K. Mulligan; King, Robert C.; Stansfield, William D. (2006). A dictionary of genetics. Oxford [Oxfordshire]: Oxford University Press. p. 608. ISBN 0-19-530761-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Isbrandt D, Hopwood JJ, von Figura K, Peters C (1996). "Two novel frameshift mutations causing premature stop codons in a patient with the severe form of Maroteaux-Lamy syndrome". Hum. Mutat. 7 (4): 361–3. doi:10.1002/(SICI)1098-1004(1996)7:4<361::AID-HUMU12>3.0.CO;2-0. PMID 8723688.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Crow JF (1993). "How much do we know about spontaneous human mutation rates?". Environ. Mol. Mutagen. 21 (2): 122–9. doi:10.1002/em.2850210205. PMID 8444142.
- ^ Touriol C, Bornes S, Bonnal S, Audigier S, Prats H, Prats AC, Vagner S (2003). "Generation of protein isoform diversity by alternative initiation of translation at non-AUG codons". Biol. Cell. 95 (3–4): 169–78. doi:10.1016/S0248-4900(03)00033-9. PMID 12867081.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Maloy S (2003-11-29). "How nonsense mutations got their names". Microbial Genetics Course. San Diego State University. Retrieved 2010-03-10.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ References for the image are found in Wikimedia Commons page at: Commons:File:Notable mutations.svg#References.
- ^ Yang; et al. (1990). Michel-Beyerle, M. E. (ed.). Reaction centers of photosynthetic bacteria: Feldafing-II-Meeting. Vol. 6. Berlin: Springer-Verlag. pp. 209–18. ISBN 3-540-53420-2.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ Füllen G, Youvan DC (1994). "Genetic Algorithms and Recursive Ensemble Mutagenesis in Protein Engineering". Complexity International. 1.
- ^ Hebbel RP (2003). "Sickle hemoglobin instability: a mechanism for malarial protection". Redox Rep. 8 (5): 238–40. doi:10.1179/135100003225002826. PMID 14962356.
- ^ Varani G, McClain WH (2000). "The G x U wobble base pair. A fundamental building block of RNA structure crucial to RNA function in diverse biological systems". EMBO Rep. 1 (1): 18–23. doi:10.1093/embo-reports/kvd001. PMC 1083677. PMID 11256617.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Crick FHC, Orgel LE (1973). "Directed panspermia". Icarus. 19: 341–6. doi:10.1016/0019-1035(73)90110-3.
It is a little surprising that organisms with somewhat different codes do not coexist.
(p. 344) (Further discussion) - ^ a b c Elzanowski A, Ostell J (2008-04-07). "The Genetic Codes". National Center for Biotechnology Information (NCBI). Retrieved 2010-03-10.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Jukes TH, Osawa S (1990). "The genetic code in mitochondria and chloroplasts". Experientia. 46 (11–12): 1117–26. doi:10.1007/BF01936921. PMID 2253709.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Santos, M.A.; Tuite, M.F. (1995). "The CUG codon is decoded in vivo as serine and not leucine in Candida albicans". Nucleic Acids Research. 23 (9): 1481–6. doi:10.1093/nar/23.9.1481. PMC 306886. PMID 7784200.
- ^ Butler, G.; Rasmussen, MD; Lin, MF; Santos, MA; Sakthikumar, S; Munro, CA; Rheinbay, E; Grabherr, M; Forche, A; et al. (2009). "Evolution of pathogenicity and sexual reproduction in eight Candida genomes". Nature. 459 (7247): 657–62. doi:10.1038/nature08064. PMC 2834264. PMID 19465905.
{{cite journal}}: Explicit use of et al. in:|first=(help) - ^ Xie J, Schultz PG (2005). "Adding amino acids to the genetic repertoire". Curr Opin Chem Biol. 9 (6): 548–54. doi:10.1016/j.cbpa.2005.10.011. PMID 16260173.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wang Q, Parrish AR, Wang L (2009). "Expanding the genetic code for biological studies". Chem. Biol. 16 (3): 323–36. doi:10.1016/j.chembiol.2009.03.001. PMC 2696486. PMID 19318213.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Michael Yarus (2010) Life from an RNA world, p. 163
- ^ De Pouplana, L.R. (1998). "Genetic code origins: tRNAs older than their synthetases?". Proceedings of the National Academy of Sciences. 95 (19): 11295. doi:10.1073/pnas.95.19.11295. PMC 21636. PMID 9736730.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Freeland SJ, Hurst LD (1998). "The genetic code is one in a million". J. Mol. Evol. 47 (3): 238–48. doi:10.1007/PL00006381. PMID 9732450.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Taylor FJ, Coates D (1989). "The code within the codons". BioSystems. 22 (3): 177–87. doi:10.1016/0303-2647(89)90059-2. PMID 2650752.
- ^ Di Giulio M (1989). "The extension reached by the minimization of the polarity distances during the evolution of the genetic code". J. Mol. Evol. 29 (4): 288–93. doi:10.1007/BF02103616. PMID 2514270.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wong JT (1980). "Role of minimization of chemical distances between amino acids in the evolution of the genetic code". Proc. Natl. Acad. Sci. U.S.A. 77 (2): 1083–6. doi:10.1073/pnas.77.2.1083. PMC 348428. PMID 6928661.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Knight RD, Freeland SJ, Landweber LF (1999). "Selection, history and chemistry: the three faces of the genetic code". Trends Biochem. Sci. 24 (6): 241–7. doi:10.1016/S0968-0004(99)01392-4. PMID 10366854.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Knight RD, Landweber LF (1998). "Rhyme or reason: RNA-arginine interactions and the genetic code". Chem. Biol. 5 (9): R215–20. doi:10.1016/S1074-5521(98)90001-1. PMID 9751648.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Michael Yarus, Jeremy Joseph Widmann, Rob Knight (2009) 'RNA–Amino Acid Binding: A Stereochemical Era for the Genetic Code', Journal of Molecular Evolution, 10.1007/s00239-009-9270-1
- ^ Michael Yarus (2010) Life from an RNA world, p. 170
- ^ Brooks DJ, Fresco JR, Lesk AM, Singh M (2002). "Evolution of amino acid frequencies in proteins over deep time: inferred order of introduction of amino acids into the genetic code". Mol. Biol. Evol. 19 (10): 1645–55. PMID 12270892.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Amirnovin R (1997). "An analysis of the metabolic theory of the origin of the genetic code". J. Mol. Evol. 44 (5): 473–6. doi:10.1007/PL00006170. PMID 9115171.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Ronneberg TA, Landweber LF, Freeland SJ (2000). "Testing a biosynthetic theory of the genetic code: fact or artifact?". Proc. Natl. Acad. Sci. U.S.A. 97 (25): 13690–5. doi:10.1073/pnas.250403097. PMC 17637. PMID 11087835.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Freeland SJ, Wu T, Keulmann N (2003). "The case for an error minimizing standard genetic code". Orig Life Evol Biosph. 33 (4–5): 457–77. doi:10.1023/A:1025771327614. PMID 14604186.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Baranov PV, Venin M, Provan G (2009). "Codon size reduction as the origin of the triplet genetic code". PLoS ONE. 4 (5): e5708. doi:10.1371/journal.pone.0005708. PMC 2682656. PMID 19479032.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Witzany G. (2010) Biocommunication and Natural Genome Editing. Springer, Dordrecht
- ^ Tlusty T (2010). "A colorful origin for the genetic code: Information theory, statistical mechanics and the emergence of molecular codes". Phys. Life. Rev. doi:10.1016/j.plrev.2010.06.002. PMID 12270892.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)
Further reading
- Griffiths, Anthony J. F.; Miller, Jeffrey H.; Suzuki, David T.; Lewontin, Richard C.; Gelbart, William M. (1999). An Introduction to genetic analysis (7th ed.). San Francisco: W.H. Freeman. ISBN 0-7167-3771-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002). Molecular biology of the cell (4th ed.). New York: Garland Science. ISBN 0-8153-3218-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Lodish, Harvey F.; Berk, Arnold; Zipursky, S. Lawrence; Matsudaira, Paul; Baltimore, David; Darnell, James E. (2000). Molecular cell biology (4th ed.). San Francisco: W.H. Freeman. ISBN 0-7167-3706-X.
{{cite book}}: CS1 maint: multiple names: authors list (link)
External links
- The Genetic Codes → Genetic Code Tables
- The Codon Usage Database → Codon frequency tables for many organisms
- History of deciphering the genetic code
- Symmetries in the genetic code
