Buruli ulcer: Difference between revisions
Taking a crack at alt text - feel free to improve |
Trying to clarify the cause section - will post on talk |
||
| Line 44: | Line 44: | ||
==Cause== |
==Cause== |
||
Buruli ulcer is caused by infection of the skin with the [[bacterium]] ''[[Mycobacterium ulcerans]]''.<ref name=WHO2019/> Infections typically occur near slow-moving or stagnant bodies of water, where ''M. ulcerans'' is found in aquatic insects, mollusks, fish, and the water itself.{{Sfn|Guarner|2018|pp=1–2}} How ''M. ulcerans'' is transmitted to humans remains unclear, but somehow bacteria enter the skin and begin to grow. Ulceration is primarily caused by a bacterial toxin |
Buruli ulcer is caused by infection of the skin with the [[bacterium]] ''[[Mycobacterium ulcerans]]''.<ref name=WHO2019/> Infections typically occur near slow-moving or stagnant bodies of water, where ''M. ulcerans'' is found in aquatic insects, mollusks, fish, and the water itself.{{Sfn|Guarner|2018|pp=1–2}} How ''M. ulcerans'' is transmitted to humans remains unclear, but somehow bacteria enter the skin and begin to grow. Ulceration is primarily caused by a bacterial toxin called [[mycolactone]].{{sfn|Yotsu|Suzuki|Simmonds|Bedimo|2018|pp=247–248}} As the bacteria grow, they release mycolactone into the tissue around them. Mycolactone enters [[Host (biology)|host]] cells and prevents them from secreting proteins, eventually resulting in [[cell death]].{{efn|group=note|Specifically, mycolactone blocks the action of [[Sec61]], the core [[Protein_targeting#Protein_translocation|translocation protein]] that serves as the gateway to the [[endoplasmic reticulum]]. When Sec61 is blocked, proteins that would normally enter the endoplasmic reticulum are instead translated into the [[cytosol]], causing a pathological stress response that results in [[cell death]] by [[apoptosis]].{{sfn|Yotsu|Suzuki|Simmonds|Bedimo|2018|p=251}}}}{{sfn|Yotsu|Suzuki|Simmonds|Bedimo|2018|p=251}} Dead cells slough off, leaving the open wound characteristic of the disease.{{sfn|Yotsu|Suzuki|Simmonds|Bedimo|2018|p=251}} At the same time, mycolactone prevents dying cells from [[Cell signaling|signaling]] to activate the [[immune system]]. As a result, Buruli ulcers tend to lack infiltrating immune cells.{{sfn|Yotsu|Suzuki|Simmonds|Bedimo|2018|p=251}} Immune cells that do make it to the ulcer are killed by mycolactone, and [[histopathology|tissue examinations]] of the ulcer show a core of growing bacteria surrounded by debris from dead and dying [[neutrophil]]s (the most common immune cell).{{sfn|Röltgen|Pluschke|2020|pp=7–8}} |
||
===Transmission=== |
===Transmission=== |
||
| Line 96: | Line 96: | ||
Buruli ulcer has been the subject of scientific research since the description of ''M. ulcerans'' in 1948, and the demonstration that the bacteria could cause ulcers in laboratory animals.{{Sfn|Guarner|2018|pp=1–2}}{{sfn|MacCallum|Tolhurst|Buckle|Sissons|1948|pp=95–98, 103, 117–118}} While several animals are susceptible to ''M. ulcerans'' ulcers, mice (particularly [[BALB/c]] and [[C57BL/6]] mice) are most commonly used to model Buruli ulcer in modern laboratories.{{sfn|Bolz|Ruf|2019|pp=160–161}} Since ''M. ulcerans'' can only grow in relatively cool temperatures, mice are typically infected in furless parts of the body: the ear, tail, or footpad.{{sfn|Bolz|Ruf|2019|pp=160–161}} After injection into the mouse, bacteria double every three to four days, and the first signs of skin disease appear after three to four weeks.{{sfn|Bolz|Ruf|2019|pp=163–165}} Strains of ''M. ulcerans'' used in laboratores are less standardized than their murine hosts, with different laboratories using different strains based on convenience and accessibility.{{sfn|Bolz|Ruf|2019|pp=162–163}} Three ''M. ulcerans'' strains are particularly common: "Cu001", isolated from a person in [[Adzopé]], Côte d'Ivoire in 1996; "Mu1615", from a person in Malaysia in the 1960s; and "S1013" from someone in Cameroon in 2010.{{sfn|Bolz|Ruf|2019|pp=162–163}} Most Buruli ulcer research is focused on testing new antibiotics against the bacteria, testing vaccine candidates, and better understanding how ''M. ulcerans'' infection causes disease.{{sfn|Bolz|Ruf|2019|pp=165–168}} |
Buruli ulcer has been the subject of scientific research since the description of ''M. ulcerans'' in 1948, and the demonstration that the bacteria could cause ulcers in laboratory animals.{{Sfn|Guarner|2018|pp=1–2}}{{sfn|MacCallum|Tolhurst|Buckle|Sissons|1948|pp=95–98, 103, 117–118}} While several animals are susceptible to ''M. ulcerans'' ulcers, mice (particularly [[BALB/c]] and [[C57BL/6]] mice) are most commonly used to model Buruli ulcer in modern laboratories.{{sfn|Bolz|Ruf|2019|pp=160–161}} Since ''M. ulcerans'' can only grow in relatively cool temperatures, mice are typically infected in furless parts of the body: the ear, tail, or footpad.{{sfn|Bolz|Ruf|2019|pp=160–161}} After injection into the mouse, bacteria double every three to four days, and the first signs of skin disease appear after three to four weeks.{{sfn|Bolz|Ruf|2019|pp=163–165}} Strains of ''M. ulcerans'' used in laboratores are less standardized than their murine hosts, with different laboratories using different strains based on convenience and accessibility.{{sfn|Bolz|Ruf|2019|pp=162–163}} Three ''M. ulcerans'' strains are particularly common: "Cu001", isolated from a person in [[Adzopé]], Côte d'Ivoire in 1996; "Mu1615", from a person in Malaysia in the 1960s; and "S1013" from someone in Cameroon in 2010.{{sfn|Bolz|Ruf|2019|pp=162–163}} Most Buruli ulcer research is focused on testing new antibiotics against the bacteria, testing vaccine candidates, and better understanding how ''M. ulcerans'' infection causes disease.{{sfn|Bolz|Ruf|2019|pp=165–168}} |
||
== Notes == |
|||
{{Reflist|group=note}} |
|||
== References == |
== References == |
||
Revision as of 04:02, 9 November 2020
| Buruli ulcer | |
|---|---|
| Other names | Bairnsdale ulcer, Daintree ulcer, Mossman ulcer, Kumasi ulcer, Searls ulcer[1] |
 | |
| Buruli ulcer lesions. Top-left, an early ulcer. Top-right, a larger ulcer across the lower arm and wrist. Bottom, a large ulcer on the thigh. | |
| Specialty | Infectious disease |
| Symptoms | Area of swelling that becomes an ulcer[2] |
| Causes | Mycobacterium ulcerans[2] |
| Treatment | Rifampicin and clarithromycin[3] |
| Frequency | 3,000 to 5,000 cases reported to WHO per year[4] |
Buruli ulcer is an infectious disease characterized by the development of painless open wounds. The first sign of infection is a small painless nodule or area of swelling. Over days to weeks, this nodule can grow larger under the skin. Eventually surface skin sloughs off the expanding wound, causing an open ulcer. Deep ulcers can cause scarring of muscles and tendons, resulting in permanent disability. Ulcers most commonly affect the arms or legs, but can occur anywhere on the body.
Buruli ulcer is caused by skin infection with Mycobacterium ulcerans. The bacteria live in aquatic environments, particularly in slow-moving or stagnant water, from which they are introduced to humans. The mechanism by which M. ulcerans is transmitted from the environment to humans is not known, but may involve the bite of an aquatic animal or the infection of open wounds. Once in the skin M. ulcerans grows and releases the toxin mycolactone which blocks the normal function of cells, resulting in tissue death and immune suppression at the site of the ulcer.
The World Health Organization recommends treating Buruli ulcer with a combination of the antibiotics rifampicin and clarithromycin, both of which are taken orally. With antibiotic administration and proper wound care, small ulcers typically heal within six months. Deep ulcers and those on sensitive body sites may require surgery to remove dead tissue or repair scarred muscles or joints. Healthy skin may be grafted onto larger ulcers to promote healing. Even with proper treatment, Buruli ulcer can take months to heal. Regular cleaning and dressing of wounds aids healing and prevents secondary infections.
The World Health Organization receives between 3,000 and 5,000 reports of Buruli ulcer each year.[4] Buruli ulcer occurs in rural areas near slow-moving or stagnant water. Most cases are in sub-Saharan Africa and Australia with fewer in Japan and South America. Children are most commonly infected in Africa, while adults are most commonly affected in Australia. The first written description of Buruli ulcer is credited to Albert Ruskin Cook in 1897 at Mengo Hospital in Uganda. Fifty years later, the causative bacterium was isolated and identified by a group at Melbourne University. In 1998, the World Health Organization established the Global Buruli Ulcer Initiative to coordinate global efforts to eliminate Buruli ulcer. Both the World Health Organization and the Public Library of Science consider Buruli ulcer a neglected tropical disease.
Signs and symptoms
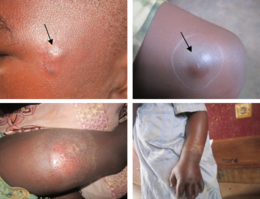
The first sign of Buruli ulcer is a painless swollen area on the arm or leg, often a small bump similar in appearance to an insect bite.[2][5] Sometimes the swollen area instead appears as a patch of firm, raised skin about three centimeters across called a "plaque"; or a more widespread swelling under the skin.[2][5] Over the course of a few weeks, the original swollen area expands to form an irregular-shaped patch of raised skin.[6][5] After about four weeks, the affected skin sloughs off leaving a painless ulcer.[2] Buruli ulcers typically have "undermined edges", with the ulcer a few centimeters wider underneath the skin than the skin wound itself.[6] In some people, the ulcer may heal on its own or remain small but linger unhealed for years.[6][7] In others, it continues to grow wider and sometimes deeper, with skin at the margin dying and sloughing off. Large ulcers may expose underlying muscle, tendon, and bone.[6] When ulcers extend into muscles and tendons, parts of these tissues can be replaced by inelastic scar tissue, immobilizing the body part and resulting in permanent disability.[6] Large exposed ulcers are often infected by other bacteria, causing the wound to smell foul.[6]
Buruli ulcers can appear anywhere on the body, but are typically on the extremities. Ulcers are most common on the lower limbs (62%) and upper limbs (24%), but can also be found on the trunk (9%), head or neck (3%), or genitals (less than 1%).[8] The World Health Organization classifies Buruli ulcer into three categories depending on the severity of its symptoms. Category I describes a single small ulcer that is less than 5 centimetres (2.0 inches). Category II describes ulcers that are larger and can be up to 15 centimetres (5.9 in). Category III is for ulcers that are larger than 15 centimeters, disseminated across the body, or include particularly sensitive sites such as the eyes, bones, joints, or genitals.[6]
Cause
Buruli ulcer is caused by infection of the skin with the bacterium Mycobacterium ulcerans.[2] Infections typically occur near slow-moving or stagnant bodies of water, where M. ulcerans is found in aquatic insects, mollusks, fish, and the water itself.[1] How M. ulcerans is transmitted to humans remains unclear, but somehow bacteria enter the skin and begin to grow. Ulceration is primarily caused by a bacterial toxin called mycolactone.[9] As the bacteria grow, they release mycolactone into the tissue around them. Mycolactone enters host cells and prevents them from secreting proteins, eventually resulting in cell death.[note 1][10] Dead cells slough off, leaving the open wound characteristic of the disease.[10] At the same time, mycolactone prevents dying cells from signaling to activate the immune system. As a result, Buruli ulcers tend to lack infiltrating immune cells.[10] Immune cells that do make it to the ulcer are killed by mycolactone, and tissue examinations of the ulcer show a core of growing bacteria surrounded by debris from dead and dying neutrophils (the most common immune cell).[11]
Transmission

It is not known how M. ulcerans is introduced to humans.[2] Human-to-human transmission is extremely rare, and Buruli ulcer is not considered contagious.[1] In areas endemic for Buruli ulcer, cases occur near stagnant bodies of water, leading to the long-standing hypothesis that M. ulcerans is somehow transmitted to humans from aquatic environments.[12] Supporting this hypothesis, M. ulcerans is widespread in aquatic environments, where it can survive as free-living or associated with other aquatic organisms.[8] Live M. ulcerans has been isolated from aquatic insects, mosses, and animal feces; and its DNA has been found in water, soil, mats of bacteria and algae, fish, crayfish, aquatic insects, and other animals that live in or near water.[12] A role for biting insects in transmission has been investigated, with particular focus on mosquitoes, giant water bugs, and Naucoridae. M. ulcerans is occasionally found in these insects, and they can sometimes transmit the bacteria in certain laboratory settings.[8] Whether these insects are regularly involved in transmission remains unclear.[1][12] Pre-existing wounds have also been implicated in disease transmission, and poor wound care is associated with a higher risk of acquiring Buruli ulcer.[13] Wearing pants and long-sleeved shirts is associated with a lower risk of Buruli ulcer, possibly by preventing insect bites or protecting wounds.[1][13]
Genetic susceptibility
While Buruli ulcer is not contagious, susceptibility sometimes runs in families, suggesting genetics could play a role in who develops disease. Severe Buruli ulcer in a Beninese family was attributed to a loss of 37 kilobases of chromosome 8 in a region that included a long non-coding RNA and was near the genes for beta-defensins.[14][15] Additionally, genome-wide association studies have linked susceptibility to Buruli ulcer to variations in six genes: SLC11A1, PRKN, NOD2, ATG16L1, iNOS, and IFNG, as well as in two long non-coding RNAs.[14]
Diagnosis
As Buruli ulcer most commonly occurs in low-resource settings, treatment is often initiated by a clinician based on signs and symptoms alone.[16] Diagnosis is then confirmed by polymerase chain reaction (PCR) to detect M. ulcerans DNA or microscopy to detect mycobacteria.[17] The gold standard test is real-time PCR to detect a DNA sequence termed IS2404 that is unique to M. ulcerans.[18] This method correctly detects M. ulcerans in 54–84% of infected people, and is highly specific to M. ulcerans.[19] For microscopy, ulcer material is typically taken by fine-needle aspiration or swabbing the edge of the ulcer, then stained with the Ziehl-Neelsen stain which makes mycobacteria visible.[17] In practice microscopy correctly detects M. ulcerans in just 30–40% of infected people, making it a relatively insensitive diagnostic test.[19]
For many bacterial infections, the gold standard for diagnosis is isolating and growing the infective organism in laboratory media. M. ulcerans can be grown in laboratory media, but its extremely slow growth rate prevents this from being used diagnostically; even under optimal growth conditions, the bacteria must grow for 9 to 12 weeks before they can be easily detected and identified.[19] An additional method of diagnosis is microscopic examination of ulcer tissue by a trained pathologist. However, this requires more invasive sampling of ulcer tissue, as well as specifically trained personnel, and so is rarely used in places where Buruli ulcer is endemic.[20]
Other ulcerative diseases can appear similarly to Buruli ulcer at its various stages. The nodule that appears early in the disease can resemble a bug bite, sebaceous cyst, lipoma, onchocerciasis (in Africa), other mycobacterial skin infections, or an enlarged lymph node.[6] Skin ulcers can resemble those caused by leishmaniasis, yaws, squamous cell carcinoma, Haemophilus ducreyi infection, and tissue death due to poor circulation.[6] More diffuse lesions can resemble cellulitis and various fungal infections of the skin.[6]
Treatment

Buruli ulcer is treated through a combination of antibiotics to kill the bacteria, and wound care or surgery to support the healing of the ulcer. The most widely used antibiotic regimen is rifampicin plus twice daily oral clarithromycin, recommended by the World Health Organization.[21][3] Several other antibiotics are sometimes used to partner with rifampicin, namely ciprofloxacin, moxifloxacin, ethambutol, amikacin, azithromycin, and levofloxacin.[21] A 2018 Cochrane review suggested that the many antibiotic combinations being used are effective treatments, but there is insufficient evidence to determine if any combination is the most effective.[22] Approximately 1 in 5 people with Buruli ulcer experience a temporary worsening of symptoms 3 to 12 weeks after they begin taking antibiotics.[23] This syndrome, called a paradoxical reaction, is more common in those with larger ulcers and ulcers on the trunk, and is more common in adults than children.[23] The paradoxical reaction in Buruli ulcer is thought to be due to the immune system responding to the wound as bacteria die and the immune-suppressing mycolactone dissipates.[23]
Small or medium-sized ulcers (WHO categories I and II) typically heal within six months of antibiotic treatment,[24] whereas larger ulcers can take over two years to fully heal.[25] Treatment sometimes includes surgery to remove necrotic ulcer tissue as well as scar tissue that can deform the muscles and joints.[23] Severe ulcers may require skin grafts to promote healing.[23] Regular wound care plays a major role in treatment, as poorly maintained wounds may become infected with other bacteria.[21]
Prevention
Buruli ulcer can be prevented by avoiding contact with aquatic environments in endemic areas; however, for those living in endemic areas, this may not be possible.[21] The risk of acquiring Buruli ulcer can be reduced by wearing long sleeves and pants, using insect repellent, and cleaning and covering any wounds as soon as they are noticed.[23] While there is no specific vaccine for Buruli ulcer, vaccination with the BCG vaccine offers temporary protection from disease.[23][26]
Epidemiology

Buruli ulcer is relatively rare, with between 3,000 and 5,000 cases reported to the World Health Organization each year.[4] Globally, most countries do not report data on Buruli ulcer to the World Health Organization, and the extent of Buruli ulcer's spread is unknown.[27][28] Even in countries that do report Buruli ulcer, health systems likely do not record each case due to insufficient reach and resources, and so the reported numbers likely underestimate the true disease prevalence.[29]
Buruli ulcer is concentrated in four areas across the globe: West Africa, coastal Australia, Japan, and South America. In West Africa, disease is predominantly reported from remote, rural communities in Benin, Côte d'Ivoire, Cameroon, and Ghana.[30] Other countries in the region also have Buruli ulcer to some degree; a 2019 systematic review of prevalence studies found a clear consensus that Buruli ulcer is present in Democratic Republic of Congo, Gabon, Liberia, Nigeria, Togo, and South Sudan, as well as "strong" or "very strong" evidence of the disease in Republic of Congo, Sierra Leone, Central African Republic, Guinea, and Uganda.[28] Buruli ulcer is also regularly reported from Australia, where it occurs in coastal clusters—two in Queensland (near Rockhampton and north of Cairns) and two in Victoria (near Bairnsdale and Melbourne).[31] It is more rarely reported from Japan and South America. Japan reports a few cases per year scattered across the main island.[32] In South America, most Buruli ulcer is reported from French Guiana, with few cases described in surrounding countries.[33] A 2019 review found "strong" evidence for the presence of Buruli ulcer in French Guiana and Peru, and "moderate" evidence in Brazil, Mexico and Suriname.[34]
Within affected countries, Buruli ulcer tends to occur in rural areas near slow-moving or stagnant water.[1] In particular, the disease tends to appear near water that has experienced human intervention, such as the building of dams or irrigation systems, flooding, or deforestation.[1] Within endemic communities, few characteristics predict who will acquire Buruli ulcer. Males and females are equally likely to be infected.[1] Ulcers can appear in people of all ages, although infections are most common among children between 5 and 15 years in West Africa, and adults over 40 in Australia and Japan.[35]
Other animals
M. ulcerans infection can cause Buruli ulcer-like lesions in some non-human animals. Natural non-human infections have only been described in coastal Victoria, near Melbourne. There, M. ulcerans positive lesions have been described in koalas, common ringtail possums, and common brushtail possums, with lesions typically on the face, limbs, and tail.[36] Ulcers have also been reported on domesticated animals, namely dogs, horses, alpacas, and a cat.[36] In laboratories several animals have been infected with M. ulcerans in an attempt to model the course of Buruli ulcer. Injections of M. ulcerans caused ulcers in several rodents (mice, guinea pigs, greater cane rats and common African rats), larger mammals (nine-banded armadillos, common brushtail possums, pigs, and Cynomolgus monkeys), and anole lizards.[37]
Society and culture
In some endemic areas, particularly rural communities in Africa, people are aware of Buruli ulcer's association with the environment, yet simultaneously associate it with witchcraft or other supernatural causes.[38] This dual understanding of disease—combined with poor access to conventional medicine—drives many to seek traditional healers as primary care.[38] Traditional healers treat Buruli ulcer with a bifurcated approach: herbs and sometimes burning or bleeding treat the physical wound; confession, ritual purification, and prohibitions on food, interpersonal contact, or sex treat the spiritual component of the disease.[39] Those with Buruli ulcer report feeling shame and experiencing social stigma that could affect their relationships, school attendance, and marriage prospects.[40]
History

While M. ulcerans may have infected humans throughout history, the first written description of Buruli ulcer is credited to a British missionary doctor, Albert R. Cook.[41][42] In 1897, at Mengo Hospital in Uganda, Cook noted several patients with slow-healing ulcers, consistent with Buruli ulcer.[43][44] The cause of these slow-healing ulcers was identified 50 years later in 1948, when Peter MacCallum, Jean Tolhurst, Glen Buckle, and H. A. Sissons at Melbourne University described a series of cases from Bairnsdale, Victoria, isolated the causative mycobacterium, and showed it could cause ulcers in laboratory rats.[1][45] Over the following decades, more cases were described in Africa, including a particularly high prevalence in Uganda's Buruli County, leading to the disease becoming more widely known as "Buruli ulcer".[43] In 1998, the World Health Organization started the Global Buruli Ulcer Initiative with the aim of coordinating global efforts to control Buruli ulcer.[43] This was followed in 2004 by World Health Organization Resolution WHA57.1 calling upon member countries to support the Global Buruli Ulcer Initiative and increase research on Buruli ulcer diagnostics and treatment.[46][47] Interest in Buruli ulcer has been encouraged by its branding as a "neglected tropical disease", first in a 2005 PLOS Medicine article, later by both the World Health Organization and PLOS Neglected Tropical Diseases.[48]
For much of the modern era, the standard treatment for Buruli ulcer was surgery to remove all affected tissue, followed by prolonged wound care.[21] Treatment dramatically improved in 2004, when the World Health Organization recommended an eight-week course of daily oral rifampicin and injected streptomycin.[21] In 2017, the World Health Organization updated its recommendation to replace streptomycin with the oral antibiotic clarithromycin.[49]
Other names
Buruli ulcer is known by several other names in different parts of the world. In southeastern Australia, it was originally called "Searls' ulcer" after the physician J. R. Searls who saw the first Australian patients at the Bairnsdale Clinic and sent material to Peter MacCallum's group at Melbourne University for further examination.[50] The disease later became more generally known as "Bairnsdale ulcer" after the district where it was described.[43] In northeastern Australia, north of Cairns, the disease is called "Daintree ulcer" or "Mossman ulcer" after the nearby Daintree River and the town of Mossman.[51][52] In Papua New Guinea, the disease is called "Kumusi ulcer" after the Kumusi River along which villages with Buruli ulcer were originally described.[53]
Research
Buruli ulcer has been the subject of scientific research since the description of M. ulcerans in 1948, and the demonstration that the bacteria could cause ulcers in laboratory animals.[1][45] While several animals are susceptible to M. ulcerans ulcers, mice (particularly BALB/c and C57BL/6 mice) are most commonly used to model Buruli ulcer in modern laboratories.[54] Since M. ulcerans can only grow in relatively cool temperatures, mice are typically infected in furless parts of the body: the ear, tail, or footpad.[54] After injection into the mouse, bacteria double every three to four days, and the first signs of skin disease appear after three to four weeks.[55] Strains of M. ulcerans used in laboratores are less standardized than their murine hosts, with different laboratories using different strains based on convenience and accessibility.[56] Three M. ulcerans strains are particularly common: "Cu001", isolated from a person in Adzopé, Côte d'Ivoire in 1996; "Mu1615", from a person in Malaysia in the 1960s; and "S1013" from someone in Cameroon in 2010.[56] Most Buruli ulcer research is focused on testing new antibiotics against the bacteria, testing vaccine candidates, and better understanding how M. ulcerans infection causes disease.[57]
Notes
- ^ Specifically, mycolactone blocks the action of Sec61, the core translocation protein that serves as the gateway to the endoplasmic reticulum. When Sec61 is blocked, proteins that would normally enter the endoplasmic reticulum are instead translated into the cytosol, causing a pathological stress response that results in cell death by apoptosis.[10]
References
Citations
- ^ a b c d e f g h i j Guarner 2018, pp. 1–2.
- ^ a b c d e f g "Buruli ulcer (Mycobacterium ulcerans infection)". World Health Organization. 21 May 2019. Retrieved 31 October 2019.
- ^ a b "Buruli ulcer (Mycobacterium ulcerans infection) – Treatment". World Health Organization. Retrieved 19 June 2020.
- ^ a b c Bravo 2019, p. 122.
- ^ a b c Yotsu et al. 2015, p. 1034.
- ^ a b c d e f g h i j Guarner 2018, pp. 3–4.
- ^ Röltgen & Pluschke 2020, p. 9.
- ^ a b c Zingue et al. 2018, pp. 10–13.
- ^ Yotsu et al. 2018, pp. 247–248.
- ^ a b c d Yotsu et al. 2018, p. 251.
- ^ Röltgen & Pluschke 2020, pp. 7–8.
- ^ a b c Yotsu et al. 2018, p. 250.
- ^ a b Jacobsen & Padgett 2010, pp. e678–e679.
- ^ a b Manry 2020, p. 3.
- ^ Vincent et al. 2018, p. e0006429.
- ^ Röltgen et al. 2019, pp. 190–191.
- ^ a b Röltgen et al. 2019, pp. 185–186.
- ^ Röltgen et al. 2019, pp. 186–187.
- ^ a b c Guarner 2018, pp. 4–6.
- ^ Röltgen et al. 2019, pp. 189–190.
- ^ a b c d e f Yotsu et al. 2018, pp. 251–252.
- ^ Yotsu, Richardson & Ishii 2018, p. 3.
- ^ a b c d e f g Guarner 2018, pp. 6–7.
- ^ Kpeli & Yeboah-Manu 2019, pp. 227–228.
- ^ Kpeli & Yeboah-Manu 2019, pp. 235–236.
- ^ Zimmerman, Finn & Curtis 2018, pp. 682–684.
- ^ Simpson et al. 2019, pp. e912–e913.
- ^ a b Simpson et al. 2019, pp. e917–e918.
- ^ Zingue et al. 2018, pp. 30–31.
- ^ Tabah et al. 2019, pp. 51–54.
- ^ Johnson 2019, pp. 62–63.
- ^ Suzuki et al. 2019, pp. 87–88.
- ^ Couppié et al. 2019, pp. 77–78.
- ^ Simpson et al. 2019, pp. e918.
- ^ Yotsu et al. 2015, pp. 1033–1034.
- ^ a b Bolz & Ruf 2019, p. 159.
- ^ Bolz & Ruf 2019, pp. 160, 168–173.
- ^ a b Tabah et al. 2019, p. 44.
- ^ Nichter 2019, p. 258.
- ^ Nichter 2019, pp. 256–258.
- ^ Zingue et al. 2018, pp. 4–8.
- ^ van der Werf et al. 2005, p. 2.
- ^ a b c d Röltgen & Pluschke 2019, pp. 1–2.
- ^ "Mengo Hospital medical notes – 1897". British Library. 2017. Retrieved 5 June 2020.
- ^ a b MacCallum et al. 1948, pp. 95–98, 103, 117–118.
- ^ Working to overcome the global impact of neglected tropical diseases: First WHO report on neglected tropical diseases. World Health Organization. 2010. p. 62. ISBN 9789241564090.
- ^ "WHA57.1 – Surveillance and control of Mycobacterium ulcerans disease (Buruli ulcer)" (PDF). World Health Organization. May 2004. Retrieved 14 June 2020.
- ^ Hotez et al. 2020, pp. 1–3.
- ^ Tabah et al. 2019, p. 50.
- ^ Meyers 2007, p. 1.
- ^ O'Brien et al. 2014, p. 267.
- ^ Johnson 2019, p. 64.
- ^ Igo & Murthy 1988, p. 391.
- ^ a b Bolz & Ruf 2019, pp. 160–161.
- ^ Bolz & Ruf 2019, pp. 163–165.
- ^ a b Bolz & Ruf 2019, pp. 162–163.
- ^ Bolz & Ruf 2019, pp. 165–168.
Works cited
- Bolz M, Ruf MT (April 2019). "Buruli ulcer in animals and experimental infection models". In Pluschke G, Röltgen K (eds.). Buruli ulcer: Mycobacterium ulcerans disease. Cham, Switzerland: Springer. pp. 159–181. PMID 32091701.
- Bravo FG (November 2019). "Emerging infections: mimickers of common patterns seen in dermatopathology". Modern Pathology. 33: 118–127. doi:10.1038/s41379-019-0399-1. PMID 31685961.
- Couppié P, Blaizot R, Velvin CJ, Douine M, Combe M, Nacher M, Gozlan RE (April 2019). "Mycobacterium ulcerans infection in French Guiana; current state of knowledge". In Pluschke G, Röltgen K (eds.). Buruli ulcer: Mycobacterium ulcerans disease. Cham, Switzerland: Springer. pp. 77–85.
- Guarner J (April 2018). "Buruli Ulcer: Review of a Neglected Skin Mycobacterial Disease". Journal of Clinical Microbiology. 56 (4): e01507-17. doi:10.1128/JCM.01507-17. PMC 5869816. PMID 29343539.
- Hotez PJ, Aksoy S, Brindley PJ, Kamhawi S (January 2020). "What constitutes a neglected tropical disease". PLoS Neglected Tropical Diseases. 14 (1): e0008001. doi:10.1371/journal.pntd.0008001. PMID 31999732.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Igo JD, Murthy DP (1988). "Mycobacterium ulcerans infections in Papua New Guinea: Correlation of clinical, histological, and microbiologic features". American Journal of Tropical Medicine and Hygiene. 38 (2): 391–392. doi:10.4269/ajtmh.1988.38.391. PMID 2451445.
- Jacobsen KH, Padgett JJ (August 2010). "Risk factors for Mycobacterium ulcerans infection". International Journal of Infectious Diseases. 14 (8): e677–e681. doi:10.1016/j.ijid.2009.11.013. PMID 20185351.
- Johnson PD (April 2019). "Buruli ulcer in Australia". In Pluschke G, Röltgen K (eds.). Buruli ulcer: Mycobacterium ulcerans disease. Cham, Switzerland: Springer. pp. 61–76.
- Kpeli GS, Yeboah-Manu D (April 2019). "Secondary infection of Buruli ulcer lesions". In Pluschke G, Röltgen K (eds.). Buruli ulcer: Mycobacterium ulcerans disease. Cham, Switzerland: Springer. pp. 227–239. doi:10.1007/978-3-030-11114-4_13. ISBN 978-3-030-11113-7. PMID 32091699.
- MacCallum P, Tolhurst JC, Buckle G, Sissons HA (January 1948). "A new mycobacterial infection in man". Journal of Pathology and Bacteriology. 60 (1): 93–122. doi:10.1002/path.1700600111. PMID 18876541.
- Manry J (April 2020). "Human genetics of Buruli ulcer". Human Genetics. 139 (6–7): 847–853. doi:10.1007/s00439-020-02163-1. PMID 32266523. S2CID 215405517.
- Meyers DH (July 2007). "Mycobacterium ulcerans infection: an eponymous ulcer". Medical Journal of Australia. 187 (1): 63. doi:10.5694/j.1326-5377.2007.tb01136.x. PMID 17605723.
- Nichter M (April 2019). "Social Science Contributions to BU-Focused Health Service Research in West Africa". In Pluschke G, Röltgen K (eds.). Buruli ulcer: Mycobacterium ulcerans disease. Cham, Switzerland: Springer. pp. 249–272.
- O'Brien D, Jenkin G, Buntine J, Steffen CM, McDonald A, Horne S, Friedman ND, Athan E, Huges A, Callan PP, Johnson PD (March 2014). "Treatment and prevention of Mycobacterium ulcernas infection (Buruli ulcer) in Australia: guideline update". The Medical Journal of Australia. 200 (5): 267–270. doi:10.5694/mja13.11331. PMID 24641151.
- Röltgen K, Cruz I, Ndung'u JM, Pluschke G (2019). "Laboratory Diagnosis of Buruli Ulcer: Challenges and Future Perspectives". In Pluschke G, Röltgen K (eds.). Buruli Ulcer: Mycobacterium Ulcerans Disease (PDF). Cham, Switzerland: Springer. pp. 183–202. ISBN 978-3-030-11114-4. Retrieved 3 November 2020.
- Röltgen K, Pluschke G (2019). "Buruli ulcer: history and disease". In Pluschke G, Röltgen K (eds.). Buruli Ulcer: Mycobacterium Ulcerans Disease. Cham, Switzerland: Springer. pp. 1–41. ISBN 978-3-030-11114-4.
- Röltgen K, Pluschke G (May 2020). "Buruli ulcer: the efficacy of innate immune defense may be a key determinant for the outcome of infection with Mycobacterium ulcerans". Frontiers in Microbiology. 11: 1018. PMC 7261859. PMID 32523571.
- Simpson H, Deribe K, Tabah EN, Peters A, Maman I, Frimpong M, Ampadu E, Phillips R, Sanderson P, Pullan RL, Cano J (July 2019). "Mapping the global distribution of Buruli ulcer: a systematic review with evidence consensus". Lancet Global Health. 7 (7): e912–e922. doi:10.1016/S2214-109X(19)30171-8. PMID 31200890.
- Suzuki K, Luo Y, Miyamoto Y, Murase C, Mikami-Sugawara M, Yotsu RR, Ishii N (2019). "Buruli ulcer in Japan". In Pluschke G, Röltgen K (eds.). Buruli Ulcer: Mycobacterium Ulcerans Disease. Cham, Switzerland: Springer. pp. 87–105. ISBN 978-3-030-11114-4.
- Tabah EN, Johnson CR, Degnonvi H, Pluschke G, Röltgen K (2019). "Buruli ulcer in Africa". In Pluschke G, Röltgen K (eds.). Buruli Ulcer: Mycobacterium Ulcerans Disease. Cham, Switzerland: Springer. pp. 43–60. ISBN 978-3-030-11114-4.
- Vincent QB, Belkadi A, Fayard C, Marion E, Adeye A, Ardant MF, et al. (April 2018). "Microdeletion on chromosome 8p23.1 in a familial form of severe Buruli ulcer". PLOS Neglected Tropical Diseases. 12 (4): e0006429. doi:10.1371/journal.pntd.0006429. PMC 5945055. PMID 29708969.
- van der Werf TS, Stienstra Y, Johnson RC, Phillips R, Adjei O, Fleischer B, Wansbrough-Jones MW, Johnson PD, Portaels F, van der Graaf WT, Asiedu K (October 2005). "Mycobacterium ulcerans disease". Bulletin of the World Health Organization. 83 (10): 785–791. PMC 2626418. PMID 16283056.
- Yotsu RR, Murase C, Sugawara M, Suzuki K, Nakanaga K, Ishii N, Asiedu K (September 2015). "Revisiting Buruli Ulcer". The Journal of Dermatology. 42 (11): 1033–41. doi:10.1111/1346-8138.13049. PMID 26332541.
- Yotsu RR, Suzuki K, Simmonds RE, Bedimo R, Ablordey A, Yeboah-Manu D, Phillips R, Asiedu K (September 2018). "Buruli Ulcer: a Review of the Current Knowledge". Current Tropical Medicine Reports. 5 (4): 247–256. doi:10.1007/s40475-018-0166-2. PMC 6223704. PMID 30460172.
- Yotsu RR, Richardson M, Ishii M (August 2018). "Drugs for treating Buruli ulcer (Mycobacterium ulcerans disease)". Cochrane Database of Systematic Reviews. 8 (8): CD012118. doi:10.1002/14651858.CD012118.pub2. PMC 6513118. PMID 30136733.
- Zimmerman P, Finn A, Curtis N (July 2018). "Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis". Journal of Infectious Disease. 218 (5): 679–687. doi:10.1093/infdis/jiy207. PMID 29635431.
- Zingue D, Bouam A, Tian RB, Drancourt M (January 2018). "Buruli Ulcer, a Prototype for Ecosystem-Related Infection, Caused by Mycobacterium ulcerans". Clinical Microbiology Reviews. 31 (1): e0004-17. doi:10.1128/CMR.00045-17. PMC 5740976. PMID 29237707.
