Urea: Difference between revisions
→Discovery: Hilaire Rouelle |
|||
| Line 40: | Line 40: | ||
==Discovery== |
==Discovery== |
||
Urea was first discovered in [[urine]] in 1773 by the French chemist |
Urea was first discovered in [[urine]] in 1773 by the French chemist [[Hilaire Rouelle]]. |
||
It was the first organic compound to be artificially synthesized from inorganic starting materials, in 1828 by [[Friedrich Wöhler]], who prepared it from silver isocyanate through a reaction with ammonium chloride:<ref>{{cite book|title=Molecules That Changed The World|last=Nicolaou|first=Kyriacos Costa|authorlink=K. C. Nicolaou|coauthors=Tamsyn Montagnon|year=2008|publisher=Wiley-VCH|isbn=978-3-527-30983-2|pages=11}}</ref> |
It was the first organic compound to be artificially synthesized from inorganic starting materials, in 1828 by [[Friedrich Wöhler]], who prepared it from silver isocyanate through a reaction with ammonium chloride:<ref>{{cite book|title=Molecules That Changed The World|last=Nicolaou|first=Kyriacos Costa|authorlink=K. C. Nicolaou|coauthors=Tamsyn Montagnon|year=2008|publisher=Wiley-VCH|isbn=978-3-527-30983-2|pages=11}}</ref> |
||
Revision as of 15:09, 22 January 2009
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Aminomethanamide
| |||
| Other names
Carbamide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.286 | ||
| E number | E927b (glazing agents, ...) | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| (NH2)2CO | |||
| Molar mass | 60.07 g/mol | ||
| Appearance | white odourless solid | ||
| Density | 1.33·10³ kg/m³[1], solid | ||
| Melting point | 132.7 °C (406 K) decomposes | ||
| Boiling point | n.a. | ||
| 108 g/100 ml (20 °C) 167 g/100 ml (40 °C) 251 g/100 ml (60 °C) 400 g/100 ml (80 °C) 733 g/100 ml (100 °C) | |||
| Acidity (pKa) | 0.18 | ||
| Basicity (pKb) | 13.82 | ||
| Structure | |||
| 4.56 p/D | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
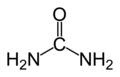
Urea is an organic compound with the chemical formula (NH2)2CO.
Urea is also known by the International Nonproprietary Name (rINN) carbamide, as established by the World Health Organization. For example, the medicinal compound hydroxyurea (old British Approved Name) is now hydroxycarbamide. Other names include carbamide resin, isourea, carbonyl diamide, and carbonyldiamine.
Discovery
Urea was first discovered in urine in 1773 by the French chemist Hilaire Rouelle.
It was the first organic compound to be artificially synthesized from inorganic starting materials, in 1828 by Friedrich Wöhler, who prepared it from silver isocyanate through a reaction with ammonium chloride:[2]
- AgNCO + NH4Cl → (NH2)2CO + AgCl
Although Wöhler was attempting to prepare ammonium cyanate, by forming urea, he inadvertently discredited vitalism, the theory that the chemicals of living organisms are fundamentally different from inanimate matter, thus starting the discipline of organic chemistry.
This discovery prompted Wöhler to write triumphantly to Berzelius:
"I must tell you that I can make urea without the use of kidneys, either man or dog. Ammonium cyanate is urea."
Wohler's historic preparation of "artificial" urea demonstrated to the scientific world that an organic compound could be synthesized, not only by a living organism, but by the working chemist. For many, then, Wöhler is considered the true father of organic chemistry.
It is found in mammalian and amphibian urine as well as in some fish. Birds and reptiles excrete uric acid, comprising a different form of nitrogen metabolism that requires less water.
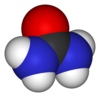
Structure
Urea is highly soluble in water and is, therefore, an efficient way for the human body to expel excess nitrogen. Its high solubility is due to extensive hydrogen bonding with water: up to eight hydrogen bonds may form - two from the oxygen atom, one from each hydrogen atom and one from each nitrogen atom.
The urea molecule is planar and retains its full molecular point symmetry, due to conjugation of one of each nitrogen's P orbital to the carbonyl double bond. Each carbonyl oxygen atom accepts four N-H-O hydrogen bonds,[citation needed] a very unusual feature for such a bond type. This dense (and energetically favourable) hydrogen bond network is probably established at the cost of efficient molecular packing: The structure is quite open, the ribbons forming tunnels with square cross-section.
Physiology
Endogenous production
The individual atoms that make up a urea molecule come from carbon dioxide, water, aspartate, and ammonia in a metabolic pathway known as the urea cycle, an anabolic process. Organisms synthesize urea from ammonia because ammonia (a common metabolic waste product) raises pH in cells to toxic levels. Therefore, urea synthesis is necessary even though it costs energy to produce. Urea is neither acidic nor basic, so it is a perfect vehicle for getting rid of nitrogen waste. Urea production occurs in the liver and is regulated by N-acetylglutamate.
In this cycle, amino groups donated by ammonia and L-aspartate are converted to urea, while L-ornithine, citrulline, L-argininosuccinate, and L-arginine act as intermediates.
Function
In humans
Urea is, in essence, a waste product. It is found and retracted from urine. However, it also plays a very important role in that it helps set up the countercurrent system in the nephrons. The countercurrent system in the nephrons allows for reabsorption of water and critical ions. Urea is reabsorbed in the inner medullary collecting ducts of the nephrons[3], thus raising the osmolarity in the medullary interstitium surrounding the thin ascending limb of the Loop of Henle. The greater the osmolarity of the medullary interstitium surrounding the thin ascending Loop of Henle, the more water will be reabsorbed out of the renal tubule back into the interstitium (and thus back into the body). Some of the urea from the medullary interstitium that helped set up the Countercurrent System will also flow back into the tubule, through urea transporter 2, into the thin ascending limb of the loop of Henle, through the collecting ducts, and eventually out of the body as a component of urine.
It is dissolved in blood (reference range of 2.5 - 7.5 mmol/liter) and excreted by the kidney as a component of urine. In addition, a small amount of urea is excreted (along with sodium chloride and water) in sweat.


Regulation
Control of urea by antidiuretic hormone allows the body to create hyperosmotic urine (urine that has more ions in it--is "more concentrated"--than that same person's blood plasma). Preventing the loss of water in this manner is important if the person's body needs to save water in order to maintain a suitable blood pressure or (more likely,) in order to maintain a suitable concentration of sodium ions in the blood plasma.
Non-humans
Most organisms have to deal with the excretion of nitrogen waste originating from protein and amino acid catabolism. In aquatic organisms the most common form of nitrogen waste is ammonia, while land-dwelling organisms convert the toxic ammonia to either urea or uric acid. In general, birds and saurian reptiles excrete uric acid, whereas the remaining species, including mammals, excrete urea. It is noteworthy that tadpoles excrete ammonia, and shift to urea production during metamorphosis.
Despite the generalization above, the pathway has been documented not only in mammals and amphibians but in many other organisms as well, including birds, invertebrates, insects, plants, yeast, fungi, and even microorganisms.
Hazards
Urea can be irritating to skin and eyes. Too high concentrations in the blood can cause damage to organs of the body. Low concentrations of urea, such as are found in typical human urine, are not dangerous with additional water ingestion within a reasonable time-frame. Many animals (to wit: dogs) have a much more concentrated urine and it contains a higher urea amount than normal human urine; this can prove dangerous as a source of liquids for consumption in a life-threatening situation (such as desert sans water).
It has been found that urea can cause algal blooms to produce toxins, and urea in runoff from fertilizers may play a role in the increase of toxic blooms.[4]
Repeated or prolonged contact with urea in fertilizer form on the skin may cause dermatitis. The substance also irritates the eyes, the skin, and the respiratory tract. The substance decomposes on heating above melting point, producing toxic gases, and reacts violently with strong oxidants, nitrites, inorganic chlorides, chlorites and perchlorates, causing fire and explosion hazard
Synthetic production
Urea is a nitrogen-containing chemical product that is produced on a scale of some 100,000,000 tons per year worldwide.
For use in industry, urea is produced from synthetic ammonia and carbon dioxide. Urea can be produced as prills, granules, flakes, pellets, crystals, and solutions.
More than 90% of world production is destined for use as a fertilizer. Urea has the highest nitrogen content of all solid nitrogenous fertilizers in common use (46.7%). Therefore, it has the lowest transportation costs per unit of nitrogen nutrient.
Urea is highly soluble in water and is, therefore, also very suitable for use in fertilizer solutions (in combination with ammonium nitrate: UAN), e.g., in 'foliar feed' fertilizers.
Solid urea is marketed as prills or granules. The advantage of prills is that, in general, they can be produced more cheaply than granules, which, because of their narrower particle size distribution, have an advantage over prills if applied mechanically to the soil. Properties such as impact strength, crushing strength, and free-flowing behaviour are, in particular, important in product handling, storage, and bulk transportation.
Commercial production
Urea is commercially produced from two raw materials, ammonia, and carbon dioxide. Large quantities of carbon dioxide are produced during the manufacture of ammonia from coal or from hydrocarbons such as natural gas and petroleum-derived raw materials. This allows direct synthesis of urea from these raw materials.
The production of urea from ammonia and carbon dioxide takes place in an equilibrium reaction, with incomplete conversion of the reactants. The various urea processes are characterized by the conditions under which urea formation takes place and the way in which unconverted reactants are further processed.
Unconverted reactants can be used for the manufacture of other products, for example ammonium nitrate or sulfate, or they can be recycled for complete conversion to urea in a total-recycle process.
Two principal reactions take place in the formation of urea from ammonia and carbon dioxide. The first reaction is exothermic:
- 2 NH3 + CO2 ↔ H2N-COONH4 (ammonium carbamate)
Whereas the second reaction is endothermic:
- H2N-COONH4 ↔ (NH2)2CO + H2O
Both reactions combined are exothermic.
The process, developed in 1922, is also called the Bosch-Meiser urea process after its discoverers.
Uses
Agricultural use
Urea is used as a nitrogen-release fertilizer, as it hydrolyses back to ammonia and carbon dioxide, but its most common impurity, biuret, must be present at less than 2%, as it impairs plant growth. It is also used in many multi-component solid fertilizer formulations. Its action of nitrogen release is due to the conditions favouring the reagent side of the equilibria, which produce urea.
Urea is usually spread at rates of between 40 and 300 kg/ha, but actual spreading rates will vary according to farm type and region. It is better to make several small to medium applications at intervals to minimise leaching losses and increase efficient use of the N applied, compared with single heavy applications. During summer, urea should be spread just before, or during rain to reduce possible losses from volatilization (process wherein nitrogen is lost to the atmosphere as ammonia gas). Urea should not be mixed for any length of time with other fertilizers, as problems of physical quality may result.
Because of the high nitrogen concentration in urea, it is very important to achieve an even spread. The application equipment must be correctly calibrated and properly used. Drilling must not occur on contact with or close to seed, due to the risk of germination damage. Urea dissolves in water for application as a spray or through irrigation systems.
In grain and cotton crops, urea is often applied at the time of the last cultivation before planting. It should be applied into or be incorporated into the soil. In high rainfall areas and on sandy soils (where nitrogen can be lost through leaching) and where good in-season rainfall is expected, urea can be side- or top-dressed during the growing season. Top-dressing is also popular on pasture and forage crops. In cultivating sugarcane, urea is side-dressed after planting, and applied to each ratoon crop.
In irrigated crops, urea can be applied dry to the soil, or dissolved and applied through the irrigation water. Urea will dissolve in its own weight in water, but it becomes increasingly difficult to dissolve as the concentration increases. Dissolving urea in water is endothermic, causing the temperature of the solution to fall when urea dissolves.
As a practical guide, when preparing urea solutions for fertigation (injection into irrigation lines), dissolve no more than 30 kg urea per 100 L water.
In foliar sprays, urea concentrations of 0.5% – 2.0% are often used in horticultural crops. As urea sprays may damage crop foliage, specific advice should be sought before use. Low-biuret grades of urea should be used if urea sprays are to be applied regularly or to sensitive horticultural crops.
Storage of urea fertilizer
Like most nitrogen products, urea absorbs moisture from the atmosphere. Therefore it should be stored either in closed/sealed bags on pallets, or, if stored in bulk, under cover with a tarpaulin. As with most solid fertilizers, it should also be stored in a cool, dry, well-ventilated area.
Industrial use
Urea has the ability to form "loose compounds", called clathrates, with many organic compounds. The organic compounds are held in channels formed by interpenetrating helices comprising of hydrogen-bonded urea molecules. This behaviour can be used to separate mixtures, and has been used in the production of aviation fuel and lubricating oils. As the helices are interconnected, all helices in a crystal must have the same "handedness". This is determined when the crystal is nucleated and can thus be forced by seeding. This property has been used to separate racemic mixtures.
Further commercial uses
- A stabilizer in nitrocellulose explosives
- A reactant in the NOx-reducing SNCR and SCR reactions in exhaust gases from combustion, for example, from power plants and diesel engines
- A component of fertilizer and animal feed, providing a relatively cheap source of nitrogen to promote growth
- A raw material for the manufacture of plastics, to be specific, urea-formaldehyde resin
- A raw material for the manufacture of various glues (urea-formaldehyde or urea-melamine-formaldehyde); the latter is waterproof and is used for marine plywood
- An alternative to rock salt in the de-icing of roadways and runways; it does not promote metal corrosion to the extent that salt does
- An additive ingredient in cigarettes, designed to enhance flavour
- A browning agent in factory-produced pretzels
- An ingredient in some hair conditioners, facial cleansers, bath oils, and lotions
- A reactant in some ready-to-use cold compresses for first-aid use, due to the endothermic reaction it creates when mixed with water
- A cloud seeding agent, along with salts, to expedite the condensation of water in clouds, producing precipitation
- An ingredient used in the past to separate paraffins, due to the ability of urea to form clathrates (also called host-guest complexes, inclusion compounds, and adducts)
- A flame-proofing agent (commonly used in dry chemical fire extinguishers as Urea-potassium bicarbonate)
- An ingredient in many tooth whitening products
- A cream to soften the skin, especially cracked skin on the bottom of one's feet
- An ingredient in dish soap.
- Along with Ammonium Phosphate, as a Yeast Nutrient, for fermentation of sugars into ethanol.
- To make potassium cyanate
- A melt agent used in re-surfacing snowboarding halfpipes and terrain park features
- An nutrient used by plankton in ocean nourishment experiments for geoengineering purposes.
Laboratory use
Urea is a powerful protein denaturant. This property can be exploited to increase the solubility of some proteins. For this application, it is used in concentrations up to 10 M. Urea is used to effectively disrupt the noncovalent bonds in proteins. Urea is an ingredient in the synthesis of urea nitrate. Urea nitrate is also a high explosive very similar to ammonium nitrate, however it may even be more powerful because of its complexity. VOD is 11,000 ft/s (3,400 m/s) to 15,420 ft/s (4,700 m/s).
Medical use
- Drug use
Urea is used in topical dermatological products to promote rehydration of the skin. If covered by an occlusive dressing, 40% urea preparations may also be used for nonsurgical debridement of nails. This drug is also used as an earwax removal aid. Like saline, urea injection is used to perform abortions. It is also the main component of an alternative medicinal treatment referred to as urine therapy.
- Clinical diagnosis
The blood urea nitrogen (BUN) test is a measure of the amount of nitrogen in the blood that comes from urea. It is used as a marker of renal function.
- Other diagnostic use
Isotopically-labeled urea (carbon-14 - radioactive, or carbon-13 - stable isotope) is used in the urea breath test, which is used to detect the presence of the bacteria Helicobacter pylori (H. pylori) in the stomach and duodenum of humans. The test detects the characteristic enzyme urease, produced by H. pylori, by a reaction that produces ammonia from urea. This increases the pH (reduces acidity) of the stomach environment around the bacteria. Similar bacteria species to H. pylori can be identified by the same test in animals such as apes, dogs, and cats (including big cats).[vague]
Textile use
Urea in textile laboratories are frequently used both in dyeing and printing as an important auxiliary, which provides solubility to the bath and retains some moisture required for the dyeing or printing process.
Ionic liquid
Choline chloride, in mixture with urea, is used as a deep eutectic solvent, a type of ionic liquid.
Automobile systems
A number of modern diesel engines, including that found on the current Mercedes-Benz ML320, use an injector containing a water-based urea solution to capture particulate emissions. The solution is injected into the exhaust system and releases ammonia. This reacts with the nitrogen oxide emissions and is converted into nitrogen and water within the catalytic converter.
Ureas
The term urea or carbamide is also used for the class of chemical compounds sharing the same functional group RR'N-CO-NRR' based on a carbonyl group flanked by two organic amine residues. They can be accessed in the laboratory by reaction of phosgene with primary or secondary amines, proceeding through an isocyanate intermediate. Non-symmetric ureas can be accessed by reaction of primary or secondary amines with an isocyanate. Example of ureas are the compounds carbamide peroxide, allantoin, and Hydantoin. Ureas are closely related to biurets and related in structure to amides, carbamates, diimides, carbodiimides, and thiocarbamides.
Reactions
Urea reacts with alcohols to form urethanes. Urea reacts with malonic esters to make barbituric acids.
References
- ^ Urea Mineral Data
- ^ Nicolaou, Kyriacos Costa (2008). Molecules That Changed The World. Wiley-VCH. p. 11. ISBN 978-3-527-30983-2.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Walter F., PhD. Boron. Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 1-4160-2328-3. Page 837
- ^ newscientist.com - US set to track toxic algal blooms
External links
- MSDS sheet on urea
- Use of urea in hand dyeing
- use of urea as an automotive reagent to reduce harmful emissions (AdBlue)