Antiarrhythmic agent: Difference between revisions
| Line 160: | Line 160: | ||
=== Class III agents === |
=== Class III agents === |
||
[[Image:Action potential Class III.svg|thumb|Class III]] |
[[Image:Action potential Class III.svg|thumb|Class III]] |
||
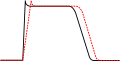
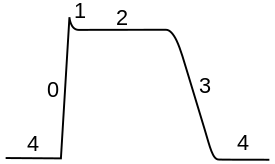
Class III agents predominantly [[Potassium channel blocker|block the potassium channels]], thereby prolonging repolarization.<ref>Lenz TL, Hilleman DE, Department of Cardiology, Creighton University, Omaha, Nebraska. Dofetilide, a New Class III Antiarrhythmic Agent. Pharmacotherapy 20(7):776–786, 2000. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10907968 Medline abstract])</ref> Since these agents do not affect the sodium channel, conduction velocity is not decreased |
Class III agents predominantly [[Potassium channel blocker|block the potassium channels]], thereby prolonging repolarization.<ref>Lenz TL, Hilleman DE, Department of Cardiology, Creighton University, Omaha, Nebraska. Dofetilide, a New Class III Antiarrhythmic Agent. Pharmacotherapy 20(7):776–786, 2000. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10907968 Medline abstract])</ref> The QT interval is prolonged with Class III agents. Since these agents do not affect the sodium channel, conduction velocity is not decreased. The prolongation of the action potential duration and refractory period, combined with the maintenance of normal conduction velocity, prevent re-entrant arrhythmias. (The re-entrant rhythm is less likely to interact with tissue that has become refractory). Drugs include: [[amiodarone]], [[ibutilide]], [[sotalol]], [[dofetilide]], and [[dronedarone]]. |
||
=== Class IV agents === |
=== Class IV agents === |
||
Revision as of 14:32, 13 October 2012
Antiarrhythmic agents are a group of pharmaceuticals that are used to suppress abnormal rhythms of the heart (cardiac arrhythmias), such as atrial fibrillation, atrial flutter, ventricular tachycardia, and ventricular fibrillation.
Many attempts have been made to classify antiarrhythmic agents. The problem arises from the fact that many of the antiarrhythmic agents have multiple modes of action, making any classification imprecise.
Singh Vaughan Williams classification
The Singh Vaughan Williams classification, introduced in 1970 based on the seminal work of Bramah N. Singh in his doctoral thesis at Oxford where Vaughan Williams was his advisor and on subsequent work by Singh and his colleagues in the United States, is one of the most widely used classification schemes for antiarrhythmic agents. This scheme classifies a drug based on the primary mechanism of its antiarrhythmic effect. However, its dependence on primary mechanism is one of the limitations of the Singh-VW classification, since many antiarrhythmic agents have multiple action mechanisms. Amiodarone, for example, has effects consistent with all of the first four classes. Another limitation is the lack of consideration within the Singh-VW classification system for the effects of drug metabolites. Procainamide—a class Ia agent whose metabolite N-acetyl procainamide (NAPA) has a class III action—is one such example. A historical limitation was that drugs such as digoxin and adenosine – important antiarrhythmic agents – had no place at all in the VW classification system. This has since been rectified by the inclusion of class V. [citation needed]
With regards to management of atrial fibrillation, Class I and III are used in rhythm control as medical cardioversion agents whilst Class II and IV are used as rate control agents.
There are five main classes in the Singh Vaughan Williams classification of antiarrhythmic agents:
- Class I agents interfere with the sodium (Na+) channel.
- Class II agents are anti-sympathetic nervous system agents. Most agents in this class are beta blockers.
- Class III agents affect potassium (K+) efflux.
- Class IV agents affect calcium channels and the AV node.
- Class V agents work by other or unknown mechanisms.
Overview table
| Class | Known as | Examples | Mechanism | Clinical uses in cardiology [1] |
|---|---|---|---|---|
| Ia | fast-channel blockers-affect QRS complex | (Na+) channel block (intermediate association/dissociation) |
| |
| Ib- Do not affect QRS complex | (Na+) channel block (fast association/dissociation) |
| ||
| Ic | (Na+) channel block (slow association/dissociation) |
| ||
| II | Beta-blockers | beta blocking Propranolol also shows some class I action |
| |
| III | K+ channel blocker
Sotalol is also a beta blocker[2] Amiodarone has Class I, II, III & IV activity |
| ||
| IV | slow-channel blockers | Ca2+ channel blocker |
| |
| V | Work by other or unknown mechanisms (Direct nodal inhibition). | Used in supraventricular arrhythmias, especially in Heart Failure with Atrial Fibrillation, contraindicated in ventricular arrhythmias. Or in the case of Magnesium Sulfate, used in Torsades de Pointes. |
Class I agents
The class I antiarrhythmic agents interfere with the sodium channel. Class I agents are grouped by what effect they have on the Na+ channel, and what effect they have on cardiac action potentials.
Class I agents are called Membrane Stabilizing agents. The 'stabilizing' word is used to describe the decrease of excitogenicity of the plasma membrane which is brought about by these agents. (Also noteworthy is that a few class II agents like propranolol also have a membrane stabilizing effect.)
Class I agents are divided into three groups (Ia, Ib and Ic) based upon their effect on the length of the action potential.[3][4]
- Ia lengthens the action potential (right shift)
- Ib shortens the action potential (left shift)
- Ic does not significantly affect the action potential (no shift)
-
Class Ia
-
Class Ib
-
Class Ic
There is a popular mnemonic to remember the class I agents:
| Class Ia | Disopyramide, Quinidine, Procainamide | Double Quarter Pounder
Police Department Questions (Procainamide, Disopyramide, Quinidine) |
| Class Ib | Lidocaine, Mexiletine, Tocainide | Lettuce, Mayo, Tomato
The Little Man (Tocainide, Lidocaine, Mexiletine) |
| Class Ic | Moricizine, Flecainide, Propafenone | More Fries Please. (note there are two "M"s in the mnemonic, but moricizine and more can clarify which is which)
For Pushing Ecstasy (Flecainide, Propafenone, Encainide) |
Class II agents
Class II agents are conventional beta blockers. They act by blocking the effects of catecholamines at the β1-adrenergic receptors, thereby decreasing sympathetic activity on the heart. These agents are particularly useful in the treatment of supraventricular tachycardias. They decrease conduction through the AV node.
Class II agents include atenolol, esmolol, propranolol, and metoprolol.
Class III agents

Class III agents predominantly block the potassium channels, thereby prolonging repolarization.[5] The QT interval is prolonged with Class III agents. Since these agents do not affect the sodium channel, conduction velocity is not decreased. The prolongation of the action potential duration and refractory period, combined with the maintenance of normal conduction velocity, prevent re-entrant arrhythmias. (The re-entrant rhythm is less likely to interact with tissue that has become refractory). Drugs include: amiodarone, ibutilide, sotalol, dofetilide, and dronedarone.
Class IV agents
Class IV agents are slow calcium channel blockers. They decrease conduction through the AV node, and shorten phase two (the plateau) of the cardiac action potential. They thus reduce the contractility of the heart, so may be inappropriate in heart failure. However, in contrast to beta blockers, they allow the body to retain adrenergic control of heart rate and contractility.
Class IV agents include verapamil and diltiazem.
Other agents ("Class V")
Since the development of the original Vaughan-Williams classification system, additional agents have been used that don't fit cleanly into categories I through IV.
Some sources use the term "Class V".[6] However, they are more frequently identified by their precise mechanism.
Agents include:
- Digoxin, which decreases conduction of electrical impulses through the AV node and increases vagal activity via its central action on the central nervous system.
- Adenosine[7]
- Magnesium sulfate,[8] which has been used for torsades de pointes.[9][10]
Sicilian Gambit classification
Another approach, known as the "Sicilian Gambit", placed a greater approach on the underlying mechanism.[11][12][13]
It presents the drugs on two axes, instead of one, and is presented in tabular form. On the Y axis, each drug is listed, in approximately the Vaughan Williams order. On the X axis, the channels, receptors, pumps, and clinical effects are listed for each drug, with the results listed in a grid. It is therefore not a true classification in that it does not aggregate drugs into categories.[14]
See also
References
- ^ Unless else specified in boxes, then ref is: Rang, H. P. Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4.
{{cite book}}: Unknown parameter|yearBGFHLKF=ignored (help) - ^ Kulmatycki KM, Abouchehade K, Sattari S, Jamali F (2001). "Drug-disease interactions: reduced beta-adrenergic and potassium channel antagonist activities of sotalol in the presence of acute and chronic inflammatory conditions in the rat". Br. J. Pharmacol. 133 (2): 286–94. doi:10.1038/sj.bjp.0704067. PMC 1572777. PMID 11350865.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Milne JR, Hellestrand KJ, Bexton RS, Burnett PJ, Debbas NM, Camm AJ (1984). "Class 1 antiarrhythmic drugs—characteristic electrocardiographic differences when assessed by atrial and ventricular pacing". Eur. Heart J. 5 (2): 99–107. PMID 6723689.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Trevor, Anthony J.; Katzung, Bertram G. (2003). Pharmacology. New York: Lange Medical Books/McGraw-Hill, Medical Publishing Division. p. 43. ISBN 0-07-139930-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Lenz TL, Hilleman DE, Department of Cardiology, Creighton University, Omaha, Nebraska. Dofetilide, a New Class III Antiarrhythmic Agent. Pharmacotherapy 20(7):776–786, 2000. (Medline abstract)
- ^ Fogoros, Richard N. (1999). Electrophysiologic testing. Oxford: Blackwell Science. p. 27. ISBN 0-632-04325-3.
- ^ Conti JB, Belardinelli L, Utterback DB, Curtis AB (1995). "Endogenous adenosine is an antiarrhythmic agent". Circulation. 91 (6): 1761–7. PMID 7882485.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Brugada P (2000). "Magnesium: an antiarrhythmic drug, but only against very specific arrhythmias". Eur. Heart J. 21 (14): 1116. doi:10.1053/euhj.2000.2142. PMID 10924290.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hoshino K, Ogawa K, Hishitani T, Isobe T, Eto Y (2004). "Optimal administration dosage of magnesium sulfate for torsades de pointes in children with long QT syndrome". J Am Coll Nutr. 23 (5): 497S–500S. PMID 15466950.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hoshino K, Ogawa K, Hishitani T, Isobe T, Etoh Y (2006). "Successful uses of magnesium sulfate for torsades de pointes in children with long QT syndrome". Pediatr Int. 48 (2): 112–7. doi:10.1111/j.1442-200X.2006.02177.x. PMID 16635167.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "The 'Sicilian Gambit'. A new approach to the classification of antiarrhythmic drugs based on their actions on arrhythmogenic mechanisms. The Task Force of the Working Group on Arrhythmias of the European Society of Cardiology". Eur. Heart J. 12 (10): 1112–31. 1991. PMID 1723682.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Vaughan Williams EM (1992). "Classifying antiarrhythmic actions: by facts or speculation". J Clin Pharmacol. 32 (11): 964–77. PMID 1474169.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Milestones in the Evolution of the Study of Arrhythmias". Retrieved 2008-07-31. [dead link]
- ^ Fogoros, Richard N. (1997). Antiarrhythmic drugs: a practical guide. Oxford: Blackwell Science. p. 49. ISBN 0-86542-532-9.