Defibrillation: Difference between revisions
No edit summary |
|||
| Line 33: | Line 33: | ||
===Change to a biphasic waveform=== |
===Change to a biphasic waveform=== |
||
Until the |
Until the mid 90s, external defibrillators delivered a Lown type waveform (see [[Bernard Lown]]) which was a heavily damped [[sinusoidal]] impulse having a mainly uniphasic characteristic. |
||
Biphasic defibrillation alternates the direction of the pulses, completing one cycle in approximately 10 milliseconds. Biphasic defibrillation was originally developed and used for implantable cardioverter-defibrillators. When applied to external defibrillators, biphasic defibrillation significantly decreases the energy level necessary for successful defibrillation, decreasing the risk of burns and [[myocardial]] damage. |
Biphasic defibrillation alternates the direction of the pulses, completing one cycle in approximately 10 milliseconds. Biphasic defibrillation was originally developed and used for implantable cardioverter-defibrillators. When applied to external defibrillators, biphasic defibrillation significantly decreases the energy level necessary for successful defibrillation, decreasing the risk of burns and [[myocardial]] damage. |
||
Revision as of 13:20, 26 November 2013
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
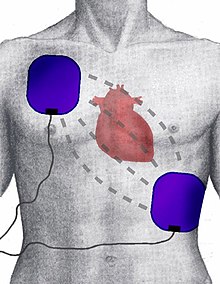
Defibrillation is a common treatment for life-threatening cardiac dysrhythmias, ventricular fibrillation and pulseless ventricular tachycardia. Defibrillation consists of delivering a therapeutic dose of electrical energy to the heart with a device called a defibrillator. This depolarizes a critical mass of the heart muscle, terminates the dysrhythmia and allows normal sinus rhythm to be reestablished by the body's natural pacemaker, in the sinoatrial node of the heart. Defibrillators can be external, transvenous, or implanted, depending on the type of device used or needed. Some external units, known as automated external defibrillators (AEDs), automate the diagnosis of treatable rhythms, meaning that lay responders or bystanders are able to use them successfully with little or no training at all.
History
Defibrillators were first demonstrated in 1899 by Jean-Louis Prévost and Frédéric Batelli, two physiologists from University of Geneva, Switzerland. They discovered that small electrical shocks could induce ventricular fibrillation in dogs, and that larger charges would reverse the condition.[1][2]
In 1933, Dr Albert Hyman, heart specialist at the Beth Davis Hospital of New York city and C. Henry Hyman, an electrical engineer, looking for an alternative to injecting powerful drugs directly into the heart, came up with an invention that used an electrical shock in place of drug injection. This invention was called the Hyman Otor where a hollow needle is used to pass an insulated wire to the heart area to deliver the electrical shock. The hollow steel needle acted as one end of the circuit and the tip of the insulated wire the other end. Whether the Hyman Otor was a success is unknown.[3]
The first use on a human was in 1947 by Claude Beck,[4] professor of surgery at Case Western Reserve University. Beck's theory was that ventricular fibrillation often occurred in hearts which were fundamentally healthy, in his terms "Hearts that are too good to die", and that there must be a way of saving them. Beck first used the technique successfully on a 14 year old boy who was being operated on for a congenital chest defect. The boy's chest was surgically opened, and manual cardiac massage was undertaken for 45 minutes until the arrival of the defibrillator. Beck used internal paddles on either side of the heart, along with procainamide, an antiarrhythmic drug, and achieved return of normal sinus rhythm.
These early defibrillators used the alternating current from a power socket, transformed from the 110-240 volts available in the line, up to between 300 and 1000 volts, to the exposed heart by way of 'paddle' type electrodes. The technique was often ineffective in reverting VF while morphological studies showed damage to the cells of the heart muscle post mortem. The nature of the AC machine with a large transformer also made these units very hard to transport, and they tended to be large units on wheels.
Closed-chest method
Until the early 1950s, defibrillation of the heart was possible only when the chest cavity was open during surgery. The technique used an alternating voltage from a 300 or greater volt source delivered to the sides of the exposed heart by 'paddle' electrodes where each electrode was a flat or slightly concave metal plate of about 40 mm diameter. The closed-chest defibrillator device which applied an alternating voltage of greater than 1000 volts, conducted by means of externally applied electrodes through the chest cage to the heart, was pioneered by Dr V. Eskin with assistance by A. Klimov in Frunze, USSR (today known as Bishkek, Kyrgyzstan) in the mid-1950s.[5]
Move to direct current

In 1959 Bernard Lown commenced research in his animal laboratory in collaboration with engineer Barouh Berkovits into an alternative technique which involved charging of a bank of capacitors to approximately 1000 volts with an energy content of 100-200 joules then delivering the charge through an inductance such as to produce a heavily damped sinusoidal wave of finite duration (~5 milliseconds) to the heart by way of paddle electrodes. This team further developed an understanding of the optimal timing of shock delivery in the cardiac cycle, enabling the application of the device to arrhythmias such as atrial fibrilllation, atrial flutter, and supraventricular tachycardias in the technique known as "cardioversion".
The Lown-Berkovits waveform, as it was known, was the standard for defibrillation until the late 1980s when numerous studies showed that a biphasic truncated waveform (BTE) was equally efficacious while requiring the delivery of lower levels of energy to produce defibrillation. An added benefit was a significant reduction in weight of the machine. The BTE waveform, combined with automatic measurement of transthoracic impedance is the basis for modern defibrillators.
Portable units become available
A major breakthrough was the introduction of portable defibrillators used out of the hospital. This was pioneered in the early 1960s by Prof. Frank Pantridge in Belfast. Today portable defibrillators are among the many very important tools carried by ambulances. They are the only proven way to resuscitate a person who has had a cardiac arrest unwitnessed by Emergency Medical Services (EMS) who is still in persistent ventricular fibrillation or ventricular tachycardia at the arrival of pre-hospital providers.
Gradual improvements in the design of defibrillators, partly based on the work developing implanted versions (see below), have led to the availability of Automated External Defibrillators. These devices can analyse the heart rhythm by themselves, diagnose the shockable rhythms, and charge to treat. This means that no clinical skill is required in their use, allowing lay people to respond to emergencies effectively.
Change to a biphasic waveform
Until the mid 90s, external defibrillators delivered a Lown type waveform (see Bernard Lown) which was a heavily damped sinusoidal impulse having a mainly uniphasic characteristic. Biphasic defibrillation alternates the direction of the pulses, completing one cycle in approximately 10 milliseconds. Biphasic defibrillation was originally developed and used for implantable cardioverter-defibrillators. When applied to external defibrillators, biphasic defibrillation significantly decreases the energy level necessary for successful defibrillation, decreasing the risk of burns and myocardial damage.
Ventricular fibrillation (VF) could be returned to normal sinus rhythm in 60% of cardiac arrest patients treated with a single shock from a monophasic defibrillator. Most biphasic defibrillators have a first shock success rate of greater than 90%.[6]
Implantable devices
A further development in defibrillation came with the invention of the implantable device, known as an implantable cardioverter-defibrillator (or ICD). This was pioneered at Sinai Hospital in Baltimore by a team that included Stephen Heilman, Alois Langer, Jack Lattuca, Morton Mower, Michel Mirowski, and Mir Imran, with the help of industrial collaborator Intec Systems of Pittsburgh.[7] Mirowski teamed up with Mower and Staewen, and together they commenced their research in 1969 but it was 11 years before they treated their first patient. Similar developmental work was carried out by Schuder and colleagues at the University of Missouri.
The work was commenced, despite doubts amongst leading experts in the field of arrhythmias and sudden death. There was doubt that their ideas would ever become a clinical reality. In 1962 Bernard Lown introduced the external DC defibrillator. This device applied a direct current from a discharging capacitor through the chest wall into the heart to stop heart fibrillation.[8] In 1972, Lown stated in the journal Circulation — "The very rare patient who has frequent bouts of ventricular fibrillation is best treated in a coronary care unit and is better served by an effective antiarrhythmic program or surgical correction of inadequate coronary blood flow or ventricular malfunction. In fact, the implanted defibrillator system represents an imperfect solution in search of a plausible and practical application."[9]
The problems to be overcome were the design of a system which would allow detection of ventricular fibrillation or ventricular tachycardia. Despite the lack of financial backing and grants, they persisted and the first device was implanted in February 1980 at Johns Hopkins Hospital by Dr. Levi Watkins, Jr. assisted by Vivien Thomas. Modern ICDs do not require a thoracotomy and possess pacing, cardioversion, and defibrillation capabilities.
The invention of implantable units is invaluable to some regular sufferers of heart problems, although they are generally only given to those people who have already had a cardiac episode.
People can live long normal lives with the devices. Many patients have multiple implants. A patient in Houston, Texas had an implant at the age of 18 in 1994 by the recent Dr. Antonio Pacifico. He was awarded "Youngest Patient with Defibrillator" in 1996. Though today these devices are implanted into small babies shortly after birth.
Types
Manual external defibrillator


The units are used in conjunction with (or more often have inbuilt) electrocardiogram readers, which the healthcare provider uses to diagnose a cardiac condition (most often fibrillation or tachycardia although there are some other rhythms which can be treated by different shocks).

The healthcare provider will then decide what charge (in joules) to use, based on proven guidelines and experience, and will deliver the shock through paddles or pads on the patient's chest. As they require detailed medical knowledge, these units are generally only found in hospitals and on some ambulances. For instance, every NHS ambulance in the United Kingdom is equipped with a manual defibrillator for use by the attending paramedics and technicians. In the United States, many advanced EMTs and all paramedics are trained to recognize lethal arrhythmias and deliver appropriate electrical therapy with a manual defibrillator when appropriate.
Manual internal defibrillator
These are the direct descendants of the work of Beck and Lown. They are virtually identical to the external version, except that the charge is delivered through internal paddles in direct contact with the heart. These are almost exclusively found in operating theatres (rooms), where the chest is likely to be open, or can be opened quickly by a surgeon.
Automated external defibrillator (AED)

These simple-to-use units are based on computer technology which is designed to analyze the heart rhythm itself, and then advise the user whether a shock is required. They are designed to be used by lay persons, who require little training to operate them correctly. They are usually limited in their interventions to delivering high joule shocks for VF (ventricular fibrillation) and VT (ventricular tachycardia) rhythms, making them generally of limited use to health professionals, who could diagnose and treat a wider range of problems with a manual or semi-automatic unit.
The automatic units also take time (generally 10–20 seconds) to diagnose the rhythm, where a professional could diagnose and treat the condition far more quickly with a manual unit.[10] These time intervals for analysis, which require stopping chest compressions, have been shown in a number of studies to have a significant negative effect on shock success.[11] This effect led to the recent change in the AHA defibrillation guideline (calling for two minutes of CPR after each shock without analyzing the cardiac rhythm) and some bodies recommend that AEDs should not be used when manual defibrillators and trained operators are available.[10]
Automated external defibrillators are generally either held by trained personnel who will attend incidents, or are public access units which can be found in places including corporate and government offices, shopping centres, airports, restaurants, casinos, hotels, sports stadiums, schools and universities, community centers, fitness centers and health clubs.[12]

The locating of a public access AED should take into account where large groups of people gather, and the risk category associated with these people, to ascertain whether the risk of a sudden cardiac arrest incident is high. For example, a center for teenage children is a particularly low risk category (as children very rarely enter heart rhythms such as VF (Ventricular Fibrillation) or VT (Ventricular Tachycardia), being generally young and fit, and the most common causes of pediatric cardiac arrest are respiratory arrest and trauma - where the heart is more likely to enter asystole or PEA, (where an AED is of no use). On the other hand, a large office building with a high ratio of males over 50 is a very high risk environment.[dubious – discuss]

In many areas, emergency services vehicles are likely to carry AEDs. EMT-Basics in most areas are not trained in manual defibrillation, and often carry an AED instead. Some ambulances carry an AED in addition to a manual unit. In addition, some police or fire service vehicles carry an AED for first responder use. Some areas have dedicated community first responders, who are volunteers tasked with keeping an AED and taking it to any victims in their area. It is also increasingly common to find AEDs on transport such as commercial airlines and cruise ships. The presence of an AED can be a particularly decisive factor in cardiac patient survival in these scenarios, as professional medical assistance may be hours away.
There are 2 types of AEDs: Fully Automated and Semi Automated. Most AEDs are semi automated. A semi automated AED automatically diagnoses heart rhythms and determines if a shock is necessary. If a shock is advised, the user must then push a button to administer the shock. A fully automated AED automatically diagnoses the heart rhythm and advises the user to stand back while the shock is automatically given. Also, some types of AEDs come with advanced features, such as a manual override or an ECG display.
In order to make them highly visible, public access AEDs often are brightly coloured, and are mounted in protective cases near the entrance of a building. When these protective cases are opened, and the defibrillator removed, some will sound a buzzer to alert nearby staff to their removal but do not necessarily summon emergency services. All trained AED operators should also know to phone for an ambulance when sending for or using an AED, as the patient will be unconscious, which always requires ambulance attendance.
Implantable cardioverter-defibrillator (ICD)
Also known as automatic internal cardiac defibrillator (AICD). These devices are implants, similar to pacemakers (and many can also perform the pacemaking function). They constantly monitor the patient's heart rhythm, and automatically administer shocks for various life threatening arrhythmias, according to the device's programming. Many modern devices can distinguish between ventricular fibrillation, ventricular tachycardia, and more benign arrhythmias like supraventricular tachycardia and atrial fibrillation. Some devices may attempt overdrive pacing prior to synchronised cardioversion. When the life threatening arrhythmia is ventricular fibrillation, the device is programmed to proceed immediately to an unsynchronized shock.
There are cases where the patient's ICD may fire constantly or inappropriately. This is considered a medical emergency, as it depletes the device's battery life, causes significant discomfort and anxiety to the patient, and in some cases may actually trigger life threatening arrhythmias. Some emergency medical services personnel are now equipped with a ring magnet to place over the device, which effectively disables the shock function of the device while still allowing the pacemaker to function (if the device is so equipped). If the device is shocking frequently, but appropriately, EMS personnel may administer sedation.
Wearable cardiac defibrillator
A development of the AICD is a portable external defibrillator that is worn like a vest.[13] The unit monitors the patient 24 hours a day and will automatically deliver a biphasic shock if needed. This device is mainly indicated in patients awaiting an implantable defibrillator. Currently[when?] only one company manufactures these and they are of limited availability.
Modelling defibrillation
The efficacy of a cardiac defibrillator is highly dependent on the position of its electrodes. Most internal defibrillators are implanted in octogenarians, but a few children need the devices. Implanting defibrillators in children is particularly difficult because children are small, will grow over time, and possess cardiac anatomy that differs from that of adults. Recently, researchers were able to create a software modeling system capable of mapping an individual’s thorax and determining the optimal position for an external or internal cardiac defibrillator.[citation needed]
With the help of pre-existing surgical planning applications, the software uses myocardial voltage gradients to predict the likelihood of successful defibrillation. According to the critical mass hypothesis, defibrillation is effective only if it produces a threshold voltage gradient in a large fraction of the myocardial mass. Usually, a gradient of three to five volts per centimeter is needed in 95% of the heart. Voltage gradients of over 60 V/cm can damage tissue. The modeling software seeks to obtain safe voltage gradients above the defibrillation threshold.
Early simulations using the software suggest that small changes in electrode positioning can have large effects on defibrillation, and despite engineering hurdles that remain, the modeling system promises to help guide the placement of implanted defibrillators in children and adults.
Recent mathematical models of defibrillation are based on the bidomain model of cardiac tissue. [14] Calculations using a realistic heart shape and fiber geometry are required to determine how cardiac tissue responds to a strong electrical shock.
Interface with the patient
The connection between the defibrillator and the patient consists of a pair of electrodes, each provided with electricity conductive gel in order to ensure a good connection and to minimize electrical resistance, also called chest impedance (despite the DC discharge) which would burn the patient. Gel may be either wet (similar in consistency to surgical lubricant) or solid (similar to gummi candy. Solid-gel is more convenient, because there is no need to clean the used gel off of patient's skin after defibrilation (the solid gel is easily lifted off of the patient). However, the use of solid-gel presents a higher risk of burns during defibrillation, since wet-gel electrodes more evenly conduct electricity into the body. Paddle electrodes, which were the first type developed, come without gel, and must have the gel applied in a separate step. Self-adhesive electrodes come prefitted with gel. There is a general division of opinion over which type of electrode is superior in hospital settings; the American Heart Association favors neither, and all modern manual defibrillators used in hospitals allow for swift switching between self-adhesive pads and traditional paddles. Each type of electrode has its merits and demerits, as discussed below.
Paddle electrodes
The most well-known type of electrode (widely depicted in films and television) is the traditional metal paddle with an insulated (usually plastic) handle. This type must be held in place on the patient's skin with approximately 25 lbs of force while a shock or a series of shocks is delivered. Paddles offer a few advantages over self-adhesive pads. Many hospitals in the United States continue the use of paddles, with disposable gel pads attached in most cases, due to the inherent speed with which these electrodes can be placed and used. This is critical during cardiac arrest, as each second of nonperfusion means tissue loss. Modern paddles allow for monitoring (electrocardiography), though in hospital situations, separate monitoring leads are often already in place.
Paddles are reusable, being cleaned after use and stored for the next patient. Gel is therefore not preapplied, and must be added before these paddles are used on the patient. Paddles are generally only found on the manual external units. Paddles require approximately 25 lbs of force to be applied while the shock is delivered.
Self-adhesive electrodes
Newer types of resuscitation electrodes are designed as an adhesive pad, which includes either solid or wet gel. These are peeled off their backing and applied to the patient's chest when deemed necessary, much the same as any other sticker. The electrodes are then connected to a defibrillator, much as the paddles would be. If defibrillation is required, the machine is charged, and the shock is delivered, without any need to apply any additional gel or to retrieve and place any paddles. Most adhesive electrodes are designed to be used not only for defibrillation, but also for transcutaneous pacing and synchronized electrical cardioversion. These adhesive pads are found on most automated and semi-automated units and are replacing paddles entirely in non-hospital settings. In hospital, for cases where cardiac arrest is likely to occur (but has not yet), self-adhesive pads may be placed prophylactically.
Pads also offer an advantage to the untrained user, and to medics working in the sub-optimal conditions of the field. Pads do not require extra leads to be attached for monitoring, and they do not require any force to be applied as the shock is delivered. Thus, adhesive electrodes minimize the risk of the operator coming into physical (and thus electrical) contact with the patient as the shock is delivered by allowing the operator to be up to several feet away. (The risk of electrical shock to others remains unchanged, as does that of shock due to operator misuse.) Self-adhesive electrodes are single-use only. They may be used for multiple shocks in a single course of treatment, but are replaced if (or in case) the patient recovers then reenters cardiac arrest.
Placement

Resuscitation electrodes are placed according to one of two schemes. The anterior-posterior scheme is the preferred scheme for long-term electrode placement. One electrode is placed over the left precordium (the lower part of the chest, in front of the heart). The other electrode is placed on the back, behind the heart in the region between the scapula. This placement is preferred because it is best for non-invasive pacing.
The anterior-apex scheme can be used when the anterior-posterior scheme is inconvenient or unnecessary. In this scheme, the anterior electrode is placed on the right, below the clavicle. The apex electrode is applied to the left side of the patient, just below and to the left of the pectoral muscle. This scheme works well for defibrillation and cardioversion, as well as for monitoring an ECG.
In popular culture
As devices that can quickly produce dramatic improvements in patient health, defibrillators are often depicted in movies, television, video games and other fictional media. Their function, however, is often exaggerated, with the defibrillator inducing a sudden, violent jerk or convulsion by the patient; in reality, although the muscles may contract, such dramatic patient presentation is rare. Similarly, medical providers are often depicted defibrillating patients with a "flat-line" ECG rhythm (also known as asystole); this is not done in real life as the heart is not restarted by the defibrillator itself. Only the cardiac arrest rhythms ventricular fibrillation and pulseless ventricular tachycardia are normally defibrillated. This is because the whole point of the exercise is to shock the patient into asystole[citation needed] and then let their heart start back beating normally. Someone who is already in asystole cannot be helped by electrical means, and usually needs urgent CPR and intravenous medication. There are also several heart rhythms that can be "shocked" when the patient is not in cardiac arrest, such as supraventricular tachycardia and ventricular tachycardia that produces a pulse; this more-complicated procedure is known as cardioversion, not defibrillation.
In Australia up until the 1990s it was quite rare for ambulances to carry defibrillators. This changed in 1990 after Australian media mogul Kerry Packer had a heart attack and, purely by chance, the ambulance that responded to the call carried a defibrillator. After recovering, Kerry Packer donated a large sum to the Ambulance Service of New South Wales in order that all ambulances in New South Wales should be fitted with a personal defibrillator, which is why defibrillators in Australia are sometimes colloquially called "Packer Whackers".[15]
See also
- Cardiopulmonary resuscitation (CPR)
- Advanced cardiac life support (ACLS)
- Cardioversion
- Automated external defibrillator
- Myocardial infarction (heart attack)
- Ambulance
- Wearable Cardioverter Defibrillator
References
- ^ J.L. Prevost and F. Batelli, "Some Effects of Electric Discharge on the Hearts of Mammals," Comptes Rendus Academie des Sciences 129 (1899), p. 1267–1268.
- ^ "Restoration of the Functions of the Heart and Central Nervous System after Complete Anemia," Nature 61 (1900), p. 532
- ^ "Self Starter For Dead Man's Heart" ,October 1933, Popular Science
- ^ "Claude Beck, defibrillation and CPR". Case Western Reserve University. Retrieved 2007-06-15.
- ^ Sov Zdravookhr Kirg. "Some results with the use of the DPA-3 defibrillator (developed by V. Ia. Eskin and A. M. Klimov) in the treatment of terminal states" (in Russian). Retrieved 2007-08-26.
- ^ Heart Smarter: EMS Implications of the 2005 AHA Guidelines for ECC & CPR pp 15-16
- ^ http://www3.interscience.wiley.com/journal/118913850/abstract?CRETRY=1&SRETRY=0
- ^ Aston, Richard (1991). Principles of Biomedical Instrumentation and Measurement: International Edition. Merrill Publishing Company. ISBN 0-02-946562-1.
- ^ "Pacemaker Failure following External Defibrillation" (PDF). Circulation: Journal of the American Heart Association. 1972. ISSN 1524-4539.
- ^ a b editors Jasmeet Soar ... (2006). Immediate Life Support: Second Edition. Resuscitation Council (UK). ISBN 1-903812-12-7.
{{cite book}}:|author=has generic name (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Eftestol T, Sunde K, Steen PA. Effects of interrupting precordial compressions on the calculated probability of defibrillation success during out-of-hospital cardiac arrest. Circulation 2002;105:2270-3
- ^ http://www.foh.dhhs.gov/Whatwedo/AED/HHSAED.ASP
- ^ "What is the LifeVest?". Zoll Lifecor. Retrieved 2009-02-09.
- ^ Trayanova N (2006). "Defibrillation of the heart: insights into mechanisms from modelling studies". Experimental Physiology. 91 (2): 323–337. doi:10.1113/expphysiol.2005.030973. PMID 16469820.
- ^ Karl Kruszelnicki (2008-08-08). "Dr Karl's Great Moments In Science, Flatline and defibrillator (Part II)". Australian Broadcasting Corporation. Retrieved 2011-12-21.
Bibliography
- Picard, André (2007-04-27). "School defibrillators could be lifesavers". The Globe and Mail. Retrieved 2008-06-20.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help)
External links
- Sudden Cardiac Arrest Foundation
- Center for Integration of Medicine and Innovative Technology
- American Red Cross: Saving a Life is as Easy as A-E-D
- FDA Heart Health Online: Automated External Defibrillator (AED)
- Resuscitation Council (UK)
- How an internal defibrillator is implanted from Children's Hospital Heart Center, Seattle.
