Propylene glycol: Difference between revisions
→Applications: copyedits |
m Updated U.S. Department of Health and Human Services link to current page (http://www.atsdr.cdc.gov/csem/csem.asp?csem=12&po=0). Prior link (http://www.atsdr.cdc.gov/csem/egpg/) went to a redirect page on the target site. |
||
| Line 157: | Line 157: | ||
* [http://www.propylene-glycol.com Propylene glycol] website |
* [http://www.propylene-glycol.com Propylene glycol] website |
||
* [http://webbook.nist.gov/cgi/cbook.cgi?ID=C57556 WebBook page for C3H8O2] |
* [http://webbook.nist.gov/cgi/cbook.cgi?ID=C57556 WebBook page for C3H8O2] |
||
* [http://www.atsdr.cdc.gov/csem/ |
* [http://www.atsdr.cdc.gov/csem/csem.asp?csem=12&po=0 ATSDR - Case Studies in Environmental Medicine: Ethylene Glycol and Propylene Glycol Toxicity] U.S. [[Department of Health and Human Services]] (public domain) |
||
* [http://chemindustry.ru/1,2-Propanediol.php Propylene Glycol - chemical product info: properties, production, applications.] |
* [http://chemindustry.ru/1,2-Propanediol.php Propylene Glycol - chemical product info: properties, production, applications.] |
||
* [http://www.sciencedaily.com/releases/2010/10/101019084607.htm Everyday Substances Increase Risk of Allergies in Children, Swedish Study Reveals] |
* [http://www.sciencedaily.com/releases/2010/10/101019084607.htm Everyday Substances Increase Risk of Allergies in Children, Swedish Study Reveals] |
||
Revision as of 16:52, 26 February 2014
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Propane-1,2-diol
| |||
| Other names
Propylene glycol; α-Propylene glycol; 1,2-Propanediol; 1,2-Dihydroxypropane; Methyl ethyl glycol (MEG); Methylethylene glycol
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.307 | ||
| E number | E1520 (additional chemicals) | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H8O2 | |||
| Molar mass | 76.095 g·mol−1 | ||
| Density | 1.036 g/cm³ | ||
| Melting point | −59 °C (−74 °F; 214 K) | ||
| Boiling point | 188.2 °C (370.8 °F; 461.3 K) | ||
| Miscible | |||
| Solubility in ethanol | Miscible | ||
| Solubility in diethyl ether | Miscible | ||
| Solubility in acetone | Miscible | ||
| Solubility in chloroform | Miscible | ||
| Thermal conductivity | 0.34 W/m-K (50% H2O @ 90 °C (194 °F)) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Propylene glycol, also called 1,2-propanediol or propane-1,2-diol, is an organic compound (a diol or double alcohol) with formula C3H8O2. It is a colorless, nearly odorless, clear, viscous liquid with a faintly sweet taste, hygroscopic and miscible with water, acetone, and chloroform.
The compound is sometimes called α-propylene glycol to distinguish it from the isomer propane-1,3-diol (β-propylene glycol).
Structure and properties
Propylene glycol is a clear, colorless and hygroscopic liquid. Propylene glycol contains an asymmetrical carbon atom, so it exists in two enantiomers. The commercial product is a racemic mixture. Pure optical isomers can be obtained by hydration of optically pure propylene oxide.[2]
The freezing point of water is depressed when mixed with propylene glycol owing to the effects of dissolution of a solute in a solvent (freezing-point depression); in general, glycols are non-corrosive, have very low volatility and very low toxicity (however, ethylene glycol is toxic to humans and many animals).
Production
Industrially, propylene glycol is produced from propylene oxide[3] (for food-grade use), and global capacity in 1990 was 900,000 tonnes per year.[4] Different manufacturers use either non-catalytic high-temperature process at 200 °C (392 °F) to 220 °C (428 °F), or a catalytic method, which proceeds at 150 °C (302 °F) to 180 °C (356 °F) in the presence of ion exchange resin or a small amount of sulfuric acid or alkali.
Final products contain 20% propylene glycol, 1.5% of dipropylene glycol and small amounts of other polypropylene glycols.[5] Further purification produces finished industrial grade or USP/JP/EP/BP grade propylene glycol that is typically 99.5% or greater. Propylene glycol can also be converted from glycerol, a biodiesel byproduct. This starting material is usually reserved for industrial use because of the noticeable odor and taste that accompany the final product.
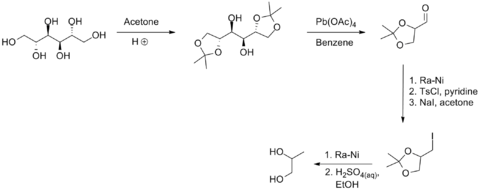
(S)-Propanediol may be synthesized from D-mannitol, through the following scheme:[6]
Applications
45% of propylene glycol produced is used as chemical feedstock for the production of unsaturated polyester resins. In this regard, propylene glycol reacts with a mixture of unsaturated maleic anhydride and isophthalic acid to give a copolymer. This partially unsaturated polymer undergoes further crosslinking to yield thermoset plastics. Related to this application, propylene glycol reacts with propylene oxide to give oligomers and polymers that are used to produce polyurethanes.[4]
Propylene glycol is considered generally recognized as safe (GRAS) by the U.S. Food and Drug Administration, and it is used as an humectant (E1520), solvent, and preservative in food and for tobacco products, as well as being one of the major ingredients of the "e-liquid" used in electronic cigarettes along with vegetable glycerin. It is also used in pharmaceutical and personal care products.[4] Propylene glycol is a solvent in many pharmaceuticals, including oral, injectable and topical formulations, such as for diazepam and lorazepam that are insoluble in water, use propylene glycol as a solvent in their clinical, injectable forms.[7]
Like ethylene glycol, propylene glycol is able to lower the freezing point of water, and so it is used as aircraft de-icing fluid.[4][8] Water-propylene glycol mixtures dyed pink to indicate the mixture is relatively nontoxic are sold under the name of RV or marine antifreeze. It is also used to winterize a vacant structure.[9] The eutectic composition/temperature is 60:40 propylene glycol:water/-60°C.[10][11] The -50°F/-45°C commercial product is, however, water rich; a typical formulation is 40:60.[12]
Propylene glycol is a minor ingredient in the oil dispersant Corexit, used in great quantities during the Deepwater Horizon oil spill.[13][14]
Propylene glycol is present in the vapor used in vaporizers.
Safety
Humans
The acute oral toxicity of propylene glycol is very low, and large quantities are required to cause perceptible health damage in humans; propylene glycol is metabolized in the human body into pyruvic acid (a normal part of the glucose-metabolism process, readily converted to energy), acetic acid (handled by ethanol-metabolism), lactic acid (a normal acid generally abundant during digestion),[15] and propionaldehyde (a potentially hazardous substance).[16][17][18]
Serious toxicity generally occurs only at plasma concentrations over 1 g/L, which requires extremely high intake over a relatively short period of time.[19] It would be nearly impossible to reach toxic levels by consuming foods or supplements, which contain at most 1 g/kg of PG. Cases of propylene glycol poisoning are usually related to either inappropriate intravenous administration or accidental ingestion of large quantities by children.[20] The potential for long-term oral toxicity is also low. In one study, in 1972, 12 rats were provided with feed containing as much as 5% PG in feed over a period of 104 weeks and they showed no apparent ill effects; no data on offspring was offered.[21] Because of its low chronic oral toxicity, propylene glycol was classified by the U. S. Food and Drug Administration as "generally recognized as safe" (GRAS) for use as a direct food additive.
Prolonged contact with propylene glycol is essentially non-irritating to the skin.[22] Undiluted propylene glycol is minimally irritating to the eye, and can produce slight transient conjunctivitis (the eye recovers after the exposure is removed). Exposure to mists may cause eye irritation, as well as upper respiratory tract irritation. Inhalation of the propylene glycol vapors appears to present no significant hazard in ordinary applications. However, limited human experience indicates that inhalation of propylene glycol mists could be irritating to some individuals.[23] It is therefore recommended that propylene glycol not be used in applications where inhalation exposure or human eye contact with the spray mists of these materials is likely, such as fogs for theatrical productions or antifreeze solutions for emergency eye wash stations.[24]
Propylene glycol does not cause sensitization and it shows no evidence of being a carcinogen or of being genotoxic.[25][26]
Adverse responses to intravenous administration of drugs that use PG as an excipient have been seen in a number of people, particularly with large dosages thereof. Responses may include "hypotension, bradycardia... QRS and T abnormalities on the ECG, arrhythmia, cardiac arrest, serum hyperosmolality, lactic acidosis, and haemolysis".[27] A high percentage (12% to 42%) of directly-injected propylene glycol is eliminated/secreted in urine unaltered depending on dosage, with the remainder appearing in its glucuronide-form. The speed of renal filtration decreases as dosage increases,[28] which may be due to propylene glycol's mild anesthetic / CNS-depressant -properties as an alcohol.[29] In one case, intravenous administration of propylene glycol-suspended nitroglycerin to an elderly man may have induced coma and acidosis.[30]
According to a 2010 study by Karlstad University, the concentrations of PGEs (counted as the sum of propylene glycol and glycol ethers) in indoor air, particularly bedroom air, has been linked to increased risk of developing numerous respiratory and immune disorders in children, including asthma, hay fever, eczema, and allergies, with increased risk ranging from 50% to 180%. This concentration has been linked to use of water-based paints and water-based system cleansers.[31][32][33]
Animals
Propylene glycol is an approved food additive for dog food under the category of animal feed and is generally recognized as safe for dogs,[34] with an LD50 of 9 mL/kg. The LD50 is higher for most laboratory animals (20 mL/kg).[35]
Similarly, propylene glycol is an approved food additive for human food as well.[36] The exception is that it is prohibited for use in food for cats due to links to Heinz body anemia.[37]
Allergic reaction
Research has suggested that individuals who cannot tolerate propylene glycol probably experience a special form of irritation, but that they only rarely develop allergic contact dermatitis. Other investigators believe that the incidence of allergic contact dermatitis to propylene glycol may be greater than 2% in patients with eczema.[38]
Patients with vulvodynia and interstitial cystitis may be especially sensitive to propylene glycol. Women suffering with yeast infections may also notice that some OTC creams can cause intense burning.[39] Post menopausal women who require the use of an estrogen cream may notice that brand name creams made with propylene glycol often create extreme, uncomfortable burning along the vulva and perianal area. Additionally, some electronic cigarette users who inhale propylene glycol vapor may experience dryness of the throat or shortness of breath . As an alternative, some suppliers will put Vegetable Glycerin in the "e-liquid" for those who are allergic (or have bad reactions) to propylene glycol.
A Swedish study published in 2010 strongly suggests a connection between airborne concentrations of propylene glycol in houses and development of asthma and allergic reactions, such as rhinitis or hives in children.[40]
Environmental
Propylene glycol is known to exert high levels of biochemical oxygen demand (BOD) during degradation in surface waters. This process can adversely affect aquatic life by consuming oxygen needed by aquatic organisms for survival. Large quantities of dissolved oxygen (DO) in the water column are consumed when microbial populations decompose propylene glycol.
Sufficient dissolved oxygen levels in surface waters are critical for the survival of fish, macroinvertebrates, and other aquatic organisms. If oxygen concentrations drop below a minimum level, organisms emigrate, if able and possible, to areas with higher oxygen levels or eventually die. This effect can drastically reduce the amount of usable aquatic habitat. Reductions in DO levels can reduce or eliminate bottom-feeder populations, create conditions that favor a change in a community’s species profile, or alter critical food-web interactions.[41]
Corexit implications
The chemical makes up 1-5% of the oil dispersant Corexit, used in great quantities during the Deepwater Horizon oil spill.[13] Corexit has come under scrutiny for possible adverse effects on marine life and humans that are exposed to it.[14]
References
- ^ Merck Index, 11th Edition, 7868
- ^ "1,2-Propanediol". ChemIndustry.ru. Retrieved 2007-12-28.
- ^ Chauvel, Alain; Lefebvre, Gilles. Petrochemical Processes. Vol. Volume 2: Major Oxygenated, Chlorinated and Nitrated Derivatives. Editions Technip. p. 26. ISBN 9782710805632.
{{cite book}}:|volume=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ a b c d Carl J. Sullivan. "Propanediols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_163. ISBN 978-3527306732.
- ^ 1,2-propanediol: chemical product info at CHEMINDUSTRY.RU
- ^ Hanessian, Stephen (1983). Total Synthesis of Natural Products: The 'Chiron' Approach. Pergamon press. p. 41. ISBN 978-0080307152.
- ^ Janusz Szajewski, MD , Warsaw Poison Control Centre (August 1991). "Propylene glycol (PIM 443)". IPCS INChem. Retrieved July 2, 2009.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ "Chemical & Engineering News: WHAT'S THAT STUFF? - AIRCRAFT DEICERS". Pubs.acs.org. 2000-07-10. Retrieved 2013-06-21.
- ^ http://www.wikihow.com/Winterize-a-Vacant-Home
- ^ http://webserver.dmt.upm.es/~isidoro/bk3/c07sol/Solution%20properties.pdf
- ^ http://windborneinpugetsound.blogspot.com/2010/08/why-does-holding-plate-work.html
- ^ http://www.chemicalspec.com/Winter_Care_4060_RV_Antifreeze.pdf
- ^ a b "Safety Data Sheet Product Corexit EC9527A" (PDF). Retrieved 2013-03-06.
- ^ a b "Chemicals Meant To Break Up BP Oil Spill Present New Environmental Concerns". ProPublica. Retrieved 2010-05-07.
- ^ Hamilton, D. J. (1890). "Gastric Dyspepsia". The Lancet. 2: 306.
- ^ "Material Safety Data Sheet Propionaldehyde MSDS". ScienceLab.com. 2010.
- ^ Miller DN, Bazzano G; Bazzano (1965). "Propanediol metabolism and its relation to lactic acid metabolism". Ann NY Acad Sci. 119 (3): 957–973. Bibcode:1965NYASA.119..957M. doi:10.1111/j.1749-6632.1965.tb47455.x. PMID 4285478.
- ^ Ruddick JA (1972). "Toxicology, metabolism, and biochemistry of 1,2-propanediol". Toxicol App Pharmacol. 21: 102–111. doi:10.1016/0041-008X(72)90032-4.
- ^ Flanagan RJ;Braithwaite RA;Brown SS;Widdop B;de Wolff FA;. The International Programme on Chemical Safety: Basic Analytical Toxicology. WHO, 1995.
- ^ National Library of Medicine;.Propylene glycol is used in antifreezes Human Toxicity Excerpts: CAS Registry Number: 57-55-6 (1,2-Propylene Glycol). Selected toxicity information from HSDB. 2005.
- ^ Gaunt, I. F.; Carpanini, F. M. B.; Grasso, P.; Lansdown, A. B. G. (1972). "Long-term toxicity of propylene glycol in rats". Food and Cosmetics Toxicology. 10 (2): 151–162. doi:10.1016/S0015-6264(72)80193-7.
- ^ Agency for Toxic Substances and Disease Registry (2008). "Addendum to the Toxicological Profile for Propylene Glycol": 7.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "DOW: Product Safety Assessment (PSA): Propylene Glycol". Retrieved 2011-11-09.
- ^ A Guide to Glycols, Dow, page 36
- ^ 1,2-Dihydroxypropane SIDS Initial Assessment Profile <http://www.chem.unep.ch/irptc/sids/OECDSIDS/57-55-6.pdf>, UNEP Publications, SIAM 11, U.S.A, January 23–26, 2001, page 21.
- ^ Title 21, U.S. Code of Federal Regulations. 1999.
- ^ Szajewski, Janusz. "Propylene Glycol (PIM 443)." 1991. 2 Jun. 2010 http://www.inchem.org/documents/pims/chemical/pim443.htm#SectionTitle:9.1%20%20Acute%20poisoning
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1097/00007691-198709000-00001, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1097/00007691-198709000-00001instead. - ^ Seidenfeld, M. A.; Hanzlik, P. J. (1932). "The general properties, actions, and toxicity of propylene glycol". J Pharmacol Exp Ther. 44: 109–121.
- ^ Demey, H.; Daelmans, R.; DeBroe, M. E.; et al. (1984). "Propylene glycol intoxication due to intravenous nitroglycerin". Lancet. 323 (8390): 1360. doi:10.1016/S0140-6736(84)91860-9.
{{cite journal}}: Explicit use of et al. in:|last4=(help) - ^ "Everyday Substances Increase Risk of Allergies in Children, Swedish Study Reveals". ScienceDaily. Oct 19, 2010.
- ^ Chemical Compounds Emitted From Common Household Paints and Cleaners Increase Risks of Asthma and Allergies in Children
- ^ Choi, Hyunok (2010-10-18). Hartl, Dominik (ed.). "Common Household Chemicals and the Allergy Risks in Pre-School Age Children". PLoS ONE. 5 (10): e13423. Bibcode:2010PLoSO...513423C. doi:10.1371/journal.pone.0013423. PMC 2956675. PMID 20976153.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ See Code of Federal Regulations: 21 CFR 582.1666
- ^ Peterson, Michael; Talcott, Patricia A. (2006). Small animal toxicology. St. Louis: Saunders Elsevier. p. 997. ISBN 0-7216-0639-3.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help)CS1 maint: multiple names: authors list (link) - ^ See Code of Federal Regulations: 21CRF184.1666
- ^ "pg_cats_latest.PDF" (PDF). Retrieved 2013-06-21.
- ^ American Medical Association, Council on Drugs (1994). AMA Drug Evaluations Annual 1994. Chicago, Illinois: American Medical Association: 1224.
{{cite journal}}: Missing or empty|title=(help) - ^ Elizabeth Vliet MD, Screaming To Be Heard: Hormonal Connections That Women Suspect and Doctors Ignore". M. Evans and Company, Inc. New York 1995
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1371/journal.pone.0013423, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1371/journal.pone.0013423instead. - ^ Environmental Impact and Benefit Assessment for Proposed Effluent Limitation Guidelines and Standards for the Airport Deicing Category, July 2009 EPA PDF
External links
- Propylene glycol website
- WebBook page for C3H8O2
- ATSDR - Case Studies in Environmental Medicine: Ethylene Glycol and Propylene Glycol Toxicity U.S. Department of Health and Human Services (public domain)
- Propylene Glycol - chemical product info: properties, production, applications.
- Everyday Substances Increase Risk of Allergies in Children, Swedish Study Reveals
- ChemSub Online: Propylene glycol



