Talk:Greenhouse effect
Template:Community article probation
| This is the talk page for discussing improvements to the Greenhouse effect article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
| Archives: Index, 1, 2, 3, 4, 5, 6, 7, 8Auto-archiving period: 60 days |
| A summary of this article appears in global warming. |
| Environment: Climate change B‑class | |||||||||||||
| |||||||||||||
| This is the talk page for discussing improvements to the Greenhouse effect article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
| Archives: Index, 1, 2, 3, 4, 5, 6, 7, 8Auto-archiving period: 60 days |
Index
|
||||||||
|
This page has archives. Sections older than 60 days may be automatically archived by Lowercase sigmabot III when more than 3 sections are present. |
Proposed rewrite of The distinction between the greenhouse effect and real greenhouses
This section is kind of rambling and redundant. I propose parring it down to
While there are some similarities between the atmospheric "greenhouse effect" and the heating mechanism of the structure from which the name is derived there are important distinctions as well. A greenhouse heats its enclosed space primarily by the prevention of convection cooling. Because a greenhouse is transparent to sunlight, solar energy passes through the enclosure unimpeded and warms the ground inside. The ground in turn warms the enclosed air which continues to heat because unlike the warm air near the surface outside, the trapped air is prevented from rising and pulling in cooler air behind it.
The climatic greenhouse effect is similar in that, like the greenhouse, our atmosphere passes sunlight nearly unimpeded. Both also limit the rate of thermal energy flowing out of the system. The heat trapping mechanisms, however, are very different. The atmosphere, which like a glass enclosure, is transparent to sunlight but it is not transparent to the energy being radiated back up from the earth's surface. Some of this radiant energy is reabsorbed by greenhouse gases and is prevented from escaping into space. This reabsorbed energy keeps the planet warmer, much the way a blanket warms us by preventing our body heat from escaping.
This is a layperson's explanation of the essential difference. I may have boiled it down to much. Comments?JPatterson (talk) 22:40, 3 February 2010 (UTC)
For a greenhouse to be warm, it have to prevent *all* means of heat transmission. That includes convection, conduction *and* radiation. If any of them is not prevented at all, the greenhouse will cool down to the environment temperature. —Preceding unsigned comment added by 213.216.225.18 (talk) 18:38, 6 April 2010 (UTC)
Major rework
Prompted somewhat by the above, I've done a major re-work of several sections of the article which are now (I contend) more accurate as well as shorter, at least in some cases William M. Connolley (talk) 21:45, 4 February 2010 (UTC)
- Looks good, other than the link to perhaps the worst definition of sensible heat I have ever laid eyes on :>). Thanks for the effort. Sorry mine fell short.JPatterson (talk) 21:51, 4 February 2010 (UTC)
- You're right. I didn't get past the first sentence without going "argh" William M. Connolley (talk) 22:34, 4 February 2010 (UTC)
- ...so I fixed it William M. Connolley (talk) 23:50, 4 February 2010 (UTC)
Is this correct?"
"In the case of the greenhouse effect the rate of radiation from the Earth to space is limited by the greenhouse". The implication here seems to be that eventually all E->S radiation escapes but that it just takes longer than would be the case sans GHG. I.e. from the ideal model:
The infrared flux density out of the top of the atmosphere:
has units so I assume is the "radiation from earth to space" above. Seems to me the only way is if
Is it the rate that is limited or the magnitude? JPatterson (talk) 00:53, 6 February 2010 (UTC)
- I have a fun suggestion for you. I'll hold off answering for a while. Get a few of the oh-so-knowledgeable sceptics (you know, the ones so expert on climate science) to try their hand at answering William M. Connolley (talk) 08:35, 6 February 2010 (UTC)
- "the ones so expert on climate science". WMC it's much more of a physics question, doncha think? --Damorbel (talk) 11:20, 6 February 2010 (UTC)
- No hints I'm afraid. Please put your answer up William M. Connolley (talk) 13:26, 6 February 2010 (UTC)
- "the ones so expert on climate science". WMC it's much more of a physics question, doncha think? --Damorbel (talk) 11:20, 6 February 2010 (UTC)
- I noodled through it on my commute home last night. If the outbound radiation suddenly dropped to zero the temperature would not drop instantly but rather decay in some sort of exponential fashion. So there is a time constant involved and it is in fact the rate of energy loss that is being decreased by the GE. I now imagine the atmosphere is rather like a large water tank, with the outbound radiative energy represented by water filling the tank at the top, and the space-bound energy represented by a hole in the bottom. The GHGs make the hole smaller which increases the time the tank would take to empty if the valve were shut off at the top.
- Still you've introduced time (rate) into the picture while all of the diagrams show static processes. It would make the article more accessible I think if we could illustrate this time flow of energy somehow.
JPatterson (talk) 17:51, 6 February 2010 (UTC)
- It appears that you have assumed that the only way that heat gets into the atmosphere is via radiation. However, convection is also important. As a result, in a cloud free sky, greenhouse gases will always emit more heat than they absorb. Also, when water vapor condenses to form clouds, some of that energy is released to space, increasing the outgoing radiation beyond the equation you presented above. In addition, the clouds themselves emit as blackbodies, adding yet another term to the equation. If the amount of heat from convection and condensation is greater than the amount released by clouds, then the greenhouse gases will still release more heat than they absorb. In that case, adding more greenhouse gases should increase the size of "the hole" by allowing the atmosphere to release energy faster. (At least, that is my understanding.) Q Science (talk) 09:45, 8 February 2010 (UTC)
- Q science you make some very weird statements "greenhouse gases will always emit more heat than they absorb" and "when water vapor condenses to form clouds, some of that energy is released to space" etc. etc. Frankly I cannot see how this sort of stuff is going to improve the article. --Damorbel (talk) 10:38, 8 February 2010 (UTC)
- Let's assume that greenhouse gases absorb 80 W/m2 via IR radiation. Let's also assume that the atmosphere absorbs 20 W/m2 via conduction/convection (called sensible heat). In this case, the atmosphere now contains an increase of 100 W/m2 of heat. Since the temperature of the atmosphere does not change (in this scenario), the greenhouse gases must release 100 W/m2, 20 W/m2 more than what they absorbed via the same mechanism. Q Science (talk) 19:14, 9 February 2010 (UTC)
- I wasn't assuming anything, just trying to get straight in my own mind whether of not GHGs reduce the rate of radiative energy flowing into space (as the article states) or its magnitude. Nor DB, was I proposing my tank analogy be placed in the article. Although I think it has some merit in helping to visualize the effect of GHG on thermal equilibrium (e.g. as the water level rises, pressure at the bottom increases, increasing the amount of outbound flow. When the outbound flow = the inbound flow, the water level (representing temperature) stabilizes. If the hole is made smaller, a new equilibrium is reached at a higher level), but I doubt one could find an RS that backs this up. JPatterson (talk) 14:21, 8 February 2010 (UTC)
- Since WMC did not respond to my challenge some time back, I won't respond to his. I do know the answer (after suitably cleaning up the poorly formed question). Those who have read my comments, primarily at the Greenhouse Gas discussion page, and understood what they read, should have no difficulty answering the above question. Here are three observations/guesses about responses. 1) Damorbel won't respond because he knows from experience that he'll get it mostly wrong. 2) WMC will get it mostly right, but his response will be so terse that it will not satisfy the discriminating reader. I think he does this because he likes to leave himself plenty of wiggle room. 3) JP's model doesn't work, in one key respect, in the limit, so he's so lost he may not even believe the correct answer. blackcloak (talk) 07:16, 2 March 2010 (UTC)
Important Omission
Williams rework does not include the important fact that GHGs emit in all directions. --Damorbel (talk) 15:36, 5 February 2010 (UTC)
- Isn't that obvious? You get it from the idealised model page, anyway William M. Connolley (talk) 20:40, 5 February 2010 (UTC)
"Isn't that obvious?" Clearly not, without it the uninformed reader might easily take the assertion that "a layer of atmosphere with greenhouses gases will radiate heat downwards" meant that downwards was the only direction of radiation. --Damorbel (talk) 15:27, 7 February 2010 (UTC)
- Feel free to add "up and" (being a layer it can't sideways, of course) William M. Connolley (talk) 17:14, 7 February 2010 (UTC)
- "Feel free to add "up and"" Er, thanks for the advice WMC but it is my contribution. The way it is written makes it clearer than your suggestion, thank you.--Damorbel (talk) 10:31, 8 February 2010 (UTC)
- Feel free to add "up and" (being a layer it can't sideways, of course) William M. Connolley (talk) 17:14, 7 February 2010 (UTC)
- Pardon my ignorance, but please explain why being a layer precludes it from radiating sideways. If you put a wrought-iron frying pan on the stove to cook pancakes, the handle also gets hot, not just the part of the pan immediately above the burner. Why should this be any different? D. F. Schmidt (talk) 16:06, 14 March 2010 (UTC)
- The atmosphere is not far from being spherically symmetrical with no large temperature gradients in the East/West axis so little heat tranfer in this direction. There is a larger temperature gradient from the equator to the poles (35K-70K over 10^4km?) so some transfer here. At the top of the homosphere (85km thick) there is the mesopause, here the temperature is about 190K so the temperature gradient is in the region of 1K/km, about 1000 times greater than that from the equator to the poles.--Damorbel (talk) 19:55, 19 March 2010 (UTC)
- Then "up and down but not sideways" is a horrible way to convey that meaning. "North and south but not east and west" is more meaningful and representative of what you mean. D. F. Schmidt (talk) 04:40, 23 March 2010 (UTC)
- Scratch that. I misread. D. F. Schmidt (talk) 04:43, 23 March 2010 (UTC)
Greenhouse gases section
I would like to see a sentence explaining the ranges. Do these represent different estimates or some sort of functional dependence on some other parameter? In other words, is C02's effect somewhere between 9% and 26% because we don't know exactly or does it vary between that range dependent on other factors? JPatterson (talk) 23:37, 8 February 2010 (UTC)
- I just noticed this comment, so I apologise for the late response and hope it's still useful. The issue is a semantic one -- since there is overlap between CO2 and other greenhouse gases, it's difficult to define the exact contribution. If you remove everything but CO2, you still get about 26% of the greenhouse effect. If you only remove CO2, it drops by about 9%. StuartH (talk) 03:17, 20 March 2010 (UTC)
'Basic mechanism' edit
I reverted the edit by Nigelj because he has made no attempt to justify it. His edit is confused and unhelpful. If User:Nigelj wants to edit someone elses work let User:Nigelj start here with an explanation of what is needed.--Damorbel (talk) 19:30, 10 February 2010 (UTC)
- Hi Damorbel. Which part of my edit do you disagree with? I don't believe it can be all of it because it has so many aspects. I have already tried to explain some WP policies and guidelines to you on my talk page so we won't go into it all again here. Just to say that from what I see, your contribution to that section was quite small, so even if you feel that you own those phrases, you should have no problem with my changes outside of that sentence. Am I right? (I'm still guessing what the actual problem is here) --Nigelj (talk) 20:36, 10 February 2010 (UTC)
- Nigelj, in the context of your changes, both Earth and Sun are proper names that should be capitalized. Damorbel, you should at least restore the spelling and grammar fixes that Nigelj made. Q Science (talk) 21:28, 10 February 2010 (UTC)
- I made the simple fixes. However, this section still needs work. Q Science (talk) 21:46, 10 February 2010 (UTC)
- Only in US English are they, and at the moment the article is an inconsistent mix of the two ( both legitimate) conventions. Also is 'Idealized greenhouse model' a proper noun too? And why is that now linked twice in adjacent paragraphs? --Nigelj (talk) 21:50, 10 February 2010 (UTC)
- Well, these articles are "typically" in US English. I left the alternate spelling of "vapor" when it was in paper titles. I left Idealized greenhouse model capitalized because it is an article title, but that may not be a valid reason. Typically, I would capitalize all 3 words in this case, but that is the style "I" follow and not from some standard. I have no problem with multiple links in large articles, but I agree that adjacent paragraphs is too frequent. Please don't read my changes as the only ones I agree with, but as the ones the must be made. Q Science (talk) 00:20, 11 February 2010 (UTC)
Thermal radiation from gases produces an omnidirectional field, this should be clear in the article. I improved WMC's revision which implied that radiation from GHS was only downwards. Any picture of the Earth in the infrared will show the GHGs glowing brightly, i.e. they are the principle way the Earth is cooled. This should be made clear in the article, your editing eliminated this .--Damorbel (talk) 21:44, 10 February 2010 (UTC)
- You're going to have to be more specific as to article wording I'm afraid. I'm perfectly aware of the physics of what you're saying and I clarified it elsewhere in the article yesterday too, despite JPat breaking that bit again today. As to your reversion, which part of "a layer of atmosphere with greenhouses gases will absorb heat being radiated upwards from lower layers, and re-radiate it in all directions, both upwards and downwards" (my words) is worse than "a layer of atmosphere with greenhouses gases will radiate heat in all directions, both upwards and downwards" (your words)? In the next sentence I made it even clearer by saying "In order to achieve thermal equilibrium, this results in a warmer surface below, in order still to radiate enough heat back out into deep space from the upper layers". I don't see how you can be finding fault with the physics of that. Please explain, in terms of article wording (not a new or separate explanation of things I'm sure we both understand.) --Nigelj (talk) 22:01, 10 February 2010 (UTC)
Nigel, the problem with your physics is that a gas like CO2 absorbs heat from a warmer source (2nd Law) e.g. the Earth's surface, and tranfers some of the heat by radiation to a cooler sink. The cooler sink may be deep space or an adjacent layer, normally higher up. Let us agree on the physics first and then sort out the wording later, OK? I will check the article to see if it is not necessary to mention the fundamental mechanism at this point. --Damorbel (talk) 10:21, 11 February 2010 (UTC)
- 'My' physics is the same as 'your' physics, as far as I can see. None of what you mention here was in this sentence in the article, nor was it altered by my edit. How long is this going to take? We still have other problems in this section that you reverted for no reason, and that Q Science has only partially fixed. It doesn't really matter (it's only a Wikipedia article after all), but this seems to be getting a bit laborious now over very little. --Nigelj (talk) 12:34, 11 February 2010 (UTC)
- If you both agree on the my/your physics, then, since you're both wrong, you both need to step aside so those who understand the process make the wording changes. Specifically, molecules of CO2, or any greenhouse gas that can accept EM power from the IR field they are bathed, will accept energy quanta (then transferring- or perhaps not- the energy to neighboring N2 and O2), provided the molecules are in a state that allows the corresponding energy transfers, no matter where the IR radiation originated (above or below, in altitude, the receiving level) and no matter what the relative temperatures are. That is is the heating up process. The cooling down side of the process also occurs when the steps are reversed. The relative rates determine the direction of temperature change. This explanation does not violate any law of physics. It may be an "inconvenient truth" to some, and an "incomprehensible truth" to others. blackcloak (talk) 05:59, 2 March 2010 (UTC)
Have you checked the Idealized greenhouse model? The link appears in the pargraph. It is very poor, weird statements like "much cooler and so radiates heat back away from itself at much longer wavelengths," I'll accept the current version of the Greenhouse effect for the present but with this idealized greenhouse model the picture is just becoming blurred, there is no reason to have an extra like the Idealized Greenhouse model.--Damorbel (talk) 21:34, 11 February 2010 (UTC)
So, what is the state of this discussion now? I intend to tidy up that section again soon. Blackcloak, I can assure you that there is no need to go into quantum mechanics to explain the greenhouse effect at this level, nor to bring in conduction between CO2, N2 and O2 within the atmosphere.
- I didn't use the term quantum mechanics. If "at this level" is your true motivation, then let's just say up front that this is an oversimplified explanation of a complicated subject and has been deliberately watered down so a 12 year old can understand most of it. As for conduction, where is 99% of the retained atmospheric heat (all sources) stored? If you can answer that question correctly, how did it get there, and how does it leave? But I do understand your point- why would anyone ever choose to read through a whole lot of extraneous material just to understand a simple concept. blackcloak (talk) 22:23, 11 March 2010 (UTC)
Damorbel, I have read it and made some contributions there as well. Now, on the subject of specific article wording suggestions, you have both been utterly mute, so I will list the reasons why I intend to make each change, then make it unless you can come up with better specific wording. Please have a look at WP:TPG to see why that preamble points are important.
I'm talking about the two paragraphs that make up the section 'Basic mechanism'
- Capitalisation and linking I understand that some people are American and so will insist on capitalising sun and earth whatever happens, so I will give up on that. 'Idealized greenhouse model', however does not need capitalising when it appears mid-sentence and it only needs wikilinking the first time it appears in this section.
- Actually, the Wikipedia style guide specifically says that Sun and Earth should be capitalized. Q Science (talk) 06:10, 11 March 2010 (UTC)
- Water becomes less I propose to replace "largely because the atmosphere is drier and water vapor - an important greenhouse gas - becomes less" with "largely because the atmosphere is drier there and water vapor is an important greenhouse gas". This is clearer without the parenthetic form and without repeating a synonym for 'drier'.
- Is the word "concentration" too complicated for you to consider? blackcloak (talk) 22:23, 11 March 2010 (UTC)
- Presentation becomes reasonable I want to replace "the presentation of the Idealized greenhouse model becomes more reasonable" with "the description given by the idealized greenhouse model becomes more realistic". This is because the words I propose mean what we are trying to say, but the words presentation and reasonable are slightly the wrong ones IMHO.
- Absorb then re-radiate I want to clarify "a layer of atmosphere with greenhouses gases will radiate heat in all directions, both upwards and downwards" by adding something about having to absorb the heat first before re-radiating it: "a layer of atmosphere with greenhouses gases will absorb heat, mostly being radiated upwards from lower layers, and re-radiate it in all directions both upwards and downwards".
- "a layer" does not absorb heat, the greenhouse gases do. How about, for part of this, just say- The greenhouse gas component of the atmosphere at any altitude absorbs heat energy arriving from all directions, and re-radiates heat energy in all directions. And then add something like- Any change in temperature at any one point in the atmosphere due to the inflow and outflow of heat energy is proportional to the net change in the heat energy at that point. "all directions" and "updownwards and downwards" is kinda redundunt (sorry, couldn't resist). And what do you mean by absorbing before re-radiating. This is not correct. The physics does not say this, and any author who does is just another source, even if properly referenced, of hogwash. That situation can only occur at absolute zero. blackcloak (talk) 22:23, 11 March 2010 (UTC)
- This of course assumes that convection, evaporation, and rain do not exits. It also ignores the fact that clear nights are colder than cloudy nights. Q Science (talk) 06:03, 11 March 2010 (UTC)
- I included the words "due to the inflow and outflow of heat energy" for these reasons, and used N's simplified way of referring to electromagnetic IR radiation. I chose not to instigate a confrontation over proper technical terminology. Afterall N has made it clear that there is no place in this article for terms like quantum mechanics. blackcloak (talk) 08:26, 12 March 2010 (UTC)
- Deep space at 2.7 K There is no need to mention the temperature of deep space in the next phrase - the greenhouse effect would work in the same fashion whatever its value. Therefore I want to change "thereby warming the surface and simultaneously cooling the atmosphere by transmitting heat to deep space at 2.7K" to "In order to achieve thermal equilibrium, this results in a warmer surface below, while radiating enough heat back out into deep space from the upper layers." It is the point where the warming is actually explained: the amount of greenhouse warming is calculated by setting total heat lost equal to total heat received, and saying that whatever had to be radiated upwards from the greenhouse layers to achieve that was also radiated downwards and so is added to the radiation received at the surface. Leaving that out and replacing it with a diversion about 2.7 K was a missed opportunity.
- Increasing the concentration presently "increases the amount of radiation, and thereby warms the surface more". I suggest "increases the amount of absorption and re-radiation, and thereby leads to a still warmer surface". This reinforces the point made above, that the greenhouse layers are in thermal equilibrium, absorbing and re-radiating heat.
- "thereby leads to" is a nonsequitur. Beyond that it's wrong. At night it leads to a cooler surface. You have to put in the words like, net power or energy movement and averaging over very long time periods in order qualify words like equilibrium. In point of fact, the atmosphere is never at thermal equilibrium. That term can only be used to describe a long time average. blackcloak (talk) 22:23, 11 March 2010 (UTC)
Now, while you preview these changes, remember that I have made all these changes before and that Damorbel reverted all of them, accusing me of "destroying an edit of mine in the process. You made no contribution in the talk pages on this, any particular reason. Wikipedia contributors should always respect and explain, you have done neither. I think respect and explain is an excellent policy, do you?" on my Talk page.
I am happy to work with suggestions for even better improvements than the ones I have proposed here, but I am not going to discuss every aspect of thermodynamics with all-comers. If you have a better wording suggestion, please make it here, and nothing else. I want to get on; this set of edits is only part of the story as we have no mention of higher and lower frequency IR radiation in here yet, and I believe that is important enough for inclusion too, after these fixes are in place. --Nigelj (talk) 21:09, 10 March 2010 (UTC)
- I don't care about the caps but your version is clearly better. The stuff about 2.7 K is distracting, and other bits were wrong William M. Connolley (talk) 22:23, 10 March 2010 (UTC)
- Saying that the "concentration" of CO2 is increasing is technically correct. However, it is the "mixing ratio" that has increased from 300 ppm to 380 ppm, not the "concentration" (moles per liter). The concentration changes with height, as the pressure goes down so does the concentration. However, the CO2 mixing ratio does not change with height until above 84km. Q Science (talk) 09:01, 12 March 2010 (UTC)
- You are correct within the limited scope of your usage of the term "concentration". Check out the sections titled "Qualitative definition" and "Mass versus volume". Note the STP default definition. Also note that Wikipedia seems to define "mixing ratio" only in the context of water vapor. One of the ways pseudoscience develops is by the non-standard usage of terms. blackcloak (talk) 22:06, 12 March 2010 (UTC)
'Basic mechanism' edit /2
What a mess has been made of this description! It is now mumbling and incoherent. What on earth does "Within the region where radiative effects are important the description given by the idealized greenhouse model becomes realistic" mean? Important - what - in social circles? And is there anything clear about "this results in a warmer surface below, in order still to radiate enough heat back out into deep space from the upper layers. Increasing the concentration of the gases increases the amount of absorption and re-radiation, and thereby leads to a still warmer surface.[7]"? If this is the best explanation that Greenhouse effect apologists can manage? It is little wonder that the general public are increasingly doubtful about the whole matter. I don't think you guys have a clue what you are talking about.
Some time ago I pointed out that the radiation field from greenhouse gases is omnidirectional, this doesn't appear any more, no discussion, no explanation, no wonder there is nothing but meaningless twaddle here.--Damorbel (talk) 20:39, 19 March 2010 (UTC)
I second that. The "Basic mechanism" section is terrible and pretty much incomprehensible. The picture is also not good. IMO the article should be flagged by an administrator until this is fixed.
140.160.160.52 (talk) —Preceding undated comment added 00:30, 22 March 2010 (UTC).
Explanation of numbers on File:Greenhouse_Effect.svg
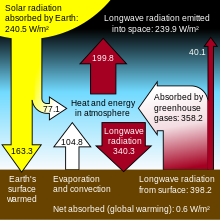
(I was about to pose this question in the talk page for the image, but there were indications that that's not the wisest place to do so.) In that file's page's text, it explains the 235 W/m2, and gives some other ratios and such. Could someone explain here or elsewhere where the rest of the numbers in the diagram come from? I see that 67 (radiation received by the earth) + 168 (radiation received by the atmosphere) = 235 (radiation delivered by the sun). 40 (lost by the earth, bypassing air) + 452 (radiated back to the air) = 492 ("Heat and energy in the atmosphere") = 324 (retaken by the earth) + 195 (released into space) - 27 (what?). And what's the number "Greenhouse gas absorption: 350" for?
Evidently, at least one of these numbers is incorrect, right? Is the Greenhouse gas value worth mentioning (how are greenhouse gases different from any other gases that keep heat in earth's system)? What's the significance of that value of 350? It seems out of place to me. What am I missing here? —Preceding unsigned comment added by D. F. Schmidt (talk • contribs) 16:54, 14 March 2010 (UTC)
- You've got 67 and 168 the wrong way round, but that doesn't matter. For the Earths sfc, from those numbers, 452+40 = 492 and 168+324 = 492 so that all fits. 324+195 = 519 = *atmos* loss. Atmos gain = 67+452 = 519, so that balances too. Solution: you've balanced the wrong numbers William M. Connolley (talk) 22:25, 14 March 2010 (UTC)
- The "452 (radiated back to the air)" is actually
- 24 thermals (basically a guess, used to balance the equations)
- 78 evaporation
- 350 surface radiation - assumed to be absorbed by greenhouse gases. However, perhaps 50% is actually absorbed by clouds.
- Earth's Annual Global Mean Energy Budget, Kiehl and Trenberth (1997), Bull. Amer. Meteor. Soc., 78, 197-208. Q Science (talk) 05:25, 20 March 2010 (UTC)
- The "452 (radiated back to the air)" is actually
Major Error in Comparison of Earth without atmosphere and Earth with Atmosphere
There is a glaring and obvious error in second paragraph. To support the idea that the earth would be -18 or -19 degrees Celsius, the contributor claims that "Earth's surface reflects about 28% of incoming sunlight," referencing the "Introduction to atmospheric chemistry" text. The problem with that citation is that the 28% value includes clouds [which reflect the lion's share of that number]. The Earth's surface albedo is much less [4% is a standard figure]. Without an atmosphere, there would be no clouds...so this becomes a false comparison. —Preceding unsigned comment added by 76.104.101.94 (talk) 18:44, 19 March 2010 (UTC)
- There is room for improvement there, I might have a go at it now, but the earth "without a greenhouse effect" is what is actually mentioned, and is a fair comparison. You're right about the surface and clouds, though. StuartH (talk) 03:10, 20 March 2010 (UTC)
- Is my edit satisfactory? You're right that it's not all the surface, so just saying the "Earth reflects" is accurate. StuartH (talk) 03:19, 20 March 2010 (UTC)
- The problem here is that the "5 degree" value is arrived at by considering the Earth as a radiative blackbody, and then a number is simply fiddled with in an effort to accommodate the clouds, but that is not the same thing as "the Earth without the greenhouse effect." For example, the "Earth without the greenhouse effect" would still lose energy through heat flux and convection, would be shielded by both clouds AND the atmosphere, etc. In other words, the formula from which the 5 degree value is obtained is not a formula that really matches the fantasy condition of starting with Earth and simply removing "The greenhouse effect." In fact the oft-quoted "-18" and "-19" degree values are contrived and have no real meaning. They ask people to pretend gases are their in one capacity and not another. If there were no greenhouse effect, it would mean no water vapor in the atmosphere [other wise you are talking about a fanciful universe where water vapor does not act like water vapor], and if there is no water vapor in the atmosphere, no clouds can exist. It's just a meaningless statement to say what would happen "without the greenhouse effect." It is far more meaningful to say what the Earth would be like "without an atmosphere."
- To put a fine point on this, take a look at one of the sources for that statistic: http://eesc.columbia.edu/courses/ees/climate/lectures/radiation/ . If you scroll down to where they get their value [-18], the actually say: "We have added a subscript e to the temperature to emphasize that this would be the temperature at the surface of the planet if it had no atmosphere." But this has no basis in reality because they obtain their value for the effective temperature [-18 degrees] by plugging in the albedo due to clouds. Obviously considering the earth "without an atmosphere" would mean not having clouds. This -18 number is a mix-and-match statistic with no meaning.
- If the earth truly had no atmosphere, it would be more like -50 degrees C [the mean temperature of the Moon], but this would not be its BLACKBODY temperature...which brings up another major problem any of this: you cannot use the blackbody temperature as a proxy for "mean temperature" for a planet that has no atmosphere. Due to the T^4 dependency on radiation, the mean temperature is very different from the blackbody temperature. [In the case of a planet about as far away from the Sun as the Earth is, that difference is about 50K !] —Preceding unsigned comment added by 76.104.101.94 (talk) 06:54, 24 March 2010 (UTC)
- Sorry to pile on...but I realized a more clear problem here. Without "the greenhouse effect" [i.e. gases in our atmosphere absorbing radiative energy], we would probably not have clouds at all, even if there were enough water vapor in the atmosphere to sustain oceans, etc. Without the greenhouse effect, the atmosphere would, of course, be completely different (and much, much colder). This is another reason why it makes no sense to use the Earth's "Albedo with Clouds" in a description of what the temperature would be like without the greenhouse effect. You are instantiating a parameter from one model into the other when the changes in the model would affect the parameter itself.76.104.101.94 (talk) 19:07, 24 March 2010 (UTC)
- The numbers are relevant and useful as an introduction to the greenhouse effect, but it's important they be presented as accurately as possible. It should not be understood as an earth "without an atmosphere" or "without greenhouse gases", which is why it is stated as "without a greenhouse effect", but it does ignore cloud and ice albedo feedbacks. The talk page is about improving the article, after all, so if you have a better phrasing for the numbers presented, it will be given due consideration. Perhaps a name other than "mean temperature" for the -19 degree calculation, or a calculation of the effective blackbody temperature of the surface (e.g. a mean temperature with a T^4 weighting or something similar) for a more valid comparison with the full blackbody temperature would be appropriate. On the other hand, with the earth's much greater rate of rotation and the heat capacity and heat redistribution capability of the oceans, I don't think the moon is a better "no greenhouse" model. StuartH (talk) 11:59, 30 March 2010 (UTC)
Maximizing Solar Heat Gain
beginning
This article on the greenhouse effects makes the claim that there are two greenhouse effects: one inside a building called a greenhouse and one outside the building called a greenhouse in a place called an atmosphere. For that claim to stand, we have to assume that all literature on the atmospheric greenhouse effect has resorted to a misnomer by calling it a greenhouse effect, since they are supposedly not the same thing. I would like to see evidence of this implied global mistake, as well as some proof showing that the definition of the greenhouse effect is not under dispute. If this cannot be demonstrated, then it is an assertion and should be called out as such or edited out.
Regarding the claim that the greenhouse effect inside a building is due to a lack of convection, this is not based on the design of actual greenhouse buildings. Many greenhouse buildings are deliberately designed for forced or natural convection[2,3]. Recommended air flow for the inside of a professionally built, commercially available greenhouse building is about 1 m/s [1]. This airflow is designed to regulate temperature and humidity, therefore the airflow can occur in the form of outside air ventilation, and/or inside-only air distribution[4].
In light of these facts, a redefinition of the greenhouse effect is in order, but redefining the greenhouse effect for buildings being due to "a lack of or the minimization of convection" instead of "no convection" or "suppressed convection" will now create a new problem since the exact rate of flow at which point the greenhouse disappears or goes away has never been defined and agreed upon in scientific literature. Also, in order for convection to be suppressed, the distance between the glass/plastic panels and the ground would have to be less than two inches, as has been demonstrated for convection cells in double-glass window panels[5].
No single experiment is ever valid unless it has been reproduced by others using the same prescribed techniques, but that is exactly what the Wood's experiment is -- a single experiment that has never been reproduced. As if that wasn't enough, there is a huge problem with the Wood's experiment, namely that it does not attempt to reproduce the conditions inside of an actual greenhouse, such as high humidity and natural or forced convection. What Wood attempted to reproduce was an oven, not a greenhouse. The Wood's experiment is clearly not a scientifically valid experiment and cannot be used to support any claim.
When designing buildings, architects attempt to minimize solar heat gain. This is the opposite of the greenhouse effect, which is to optimize or maximize solar heat gain. Any definition of the greenhouse effect should include the concept of solar heat gain. For maximum solar heat gain, you would want to keep natural convection rates high during the heating cycle -- not suppress them[6]. Only at night would you want to suppress convection in order to keep temperatures and humidity high.
[1] http://www.ces.ncsu.edu/depts/hort/greenhouse_veg/topics/gtp_pages/relhumidity.html
[2] http://cat.inist.fr/?aModele=afficheN&cpsidt=14372166. This also explains why a typical greenhouse has a sloped instead of a flat ceiling, e.g. -- to encourage convection, not suppress it.
[3] http://www.greenhouses-etc.net/ghse-fw/grnhouse_construction.htm. Here we see vents supplied with a greenhouse to deliberately induce convection, yet it does not destroy the greenhouse effect.
[4] http://www.docstoc.com/docs/20542896/Greenhouse-Heating-and-Ventilation-Systems
[5] http://en.wikipedia.org/wiki/Insulated_glazing
HY1802D (talk) 02:00, 20 March 2010 (UTC)
- The comparison between the so-called greenhouse effect and a real greenhouse is entirely spurious. The 'Greenhouse effect' explanation tries to make connection between a popular way of keeping a relatively small volume warm and a supposed global warming effect caused by the fact that some gases (the so called Greenhouse gases) absorb and emit radiation by thermal interactions (collisions, vibrations and rotations). Greenhouses are warmed and cooled by this mechanism also but the air inside them (when the windows are closed) does not take part in the general circulation of the atmosphere which, to a considerable extent, is driven by convection thus the heat from sunlight arriving during daylight hours is kept local (trapped?) by the glass walls. Yes, convection is important for heat distribution inside a real greenhouse, also it is important for keeping the world outside the greenhouse reasonably cool in sunlit conditions, it is in this sense that real greenhouses suppress convection.
- The Greenhouse effect that is supposed to have climate changing properties is said to arise from the fact that the GHGs are warmed by absorbing radiation in the infrared; it is then said that they tranfer heat back to the surface because GHGs also radiate in the infrared. This latter assertion is completely at variance with the second law of thermodynamics because the surface, globally speaking, is always warmer than the atmosphere and heat transfer is always from warmer places to cooler.
- Further, most explanations of the greenhouse effect show a higher level of radiation [1] from the GHGs to the surface that to outer space, this is at variation with the known characteristics of radiation from gases which is omnidirectional. What is worse, in the same diagram [2] GHGs are shown emitting levels of radiation that could only be achieved by a blackbody, this attribution of blackbody properties to a low density gas is quite absurd. --Damorbel (talk) 08:15, 20 March 2010 (UTC)
- While you may disagree with the numbers in the chart, do you at least agree that they are consistent? Outflow into space equals inflow from space (the sun). Inflow to the atmosphere equals outflow from the atmosphere. Inflow to the surface equals outflow from the surface. Do you believe that they have to be consistent, or are you willing to tolerate an inbalance? If you believe they are consistent (and should be) but the numbers are wrong, tell us which one/s is/are wrong, guess or choose a reasonable amount, and then tell us how you balance the net flows. Until you provide us with a cogent alternative, there's no way any knowledgeable person will ever take you seriously. As for me, I think the chart is probably fairly accurate and we can safely use it as a good starting point. Elsewhere I notice you are now using the term "globally speaking" in a sense that might be implying NET heat flow (EM radiation, IR in the present case) always moves energy from a warmer body to a cooler body (statistically, anyway). If this is the case, you are right. If this is not the case, you continue to suffer from the delusion that incessant repetition will ultimately lead to the acceptance of garbage ideas. Followers of Lyndon LaRouche believe this kind of thing. blackcloak (talk) 09:13, 20 March 2010 (UTC)
- Blackloack, no anount of radiation, blackbody or vibrating gaseous molecules can transfer any heat or thermal energy from a first body to a second that has a higher temperature, the heat always goes from the body with the higher temperature to one with a lower temperature, if you don't understand that all the rest of your physics is a waste of time and you would be extremely injury prone in a kitchen.
- History is packed with nutty inventors who have claimed this, you will find a good article about the sincere but illinformed people who pushed this idea here [3]. All explanations of the greenhouse effect are fully signed up to this (relatively) new way of challenging the second law of thermodynamics. You guys are a nice bunch of dreamers but your efforts to explain climate variability is quite contrary to any experimental or theoretical physics. You really should look at these matters as a question of thermodynamics. Or perhaps you think thermodynamics doesn't apply in climatology, just like the guys at EAU/CRU think they don't have to justify their statistical conclusions when contribuing to IPCC ARs.
- By the way, why do I have to explain the second law of thermodynamics to you, surely I am not the first? --Damorbel (talk) 12:10, 20 March 2010 (UTC)
- Try this. It partially explains why cloudy nights are warmer than clear nights.
- E = s (Ta4 - Tb4)
- The net heat flow is from warm to cold, but the rate of heat loss is reduced because cold air is a lot warmer than absolute zero. In order for the number of watts returned to the surface to be higher than the number emitted to space, it must be assumed that the atmosphere is IR opaque, that a warm lower layer is radiating toward the Earth, and that a cold upper layer is emitting toward space.
- Try this. It partially explains why cloudy nights are warmer than clear nights.
- By the way, a blackbody at 15°C emits 390 W/m2. Since 324 is about 83% of 390, it appears that 324 W/m2 is about correct for an atmosphere at 15°C, which then implies that the emission is from the lower 100 meters of the basically opaque atmosphere. Q Science (talk) 14:49, 20 March 2010 (UTC)
- So the greenhouse effect may be caused by clouds, not so much by CO2 then? The clear/cloudy night effect indeed arises from the absorption/reflection characteristics of clouds but aren't you are getting into Svensmark cosmic ray territory here, isn't this rather unusual for you?
- What do you mean "the number of watts returned"? Watts are joules per second and in your case they are apparently heating a surface that is warmer than the place the Watts are coming from. Real CRU stuff this, if the facts don't fit the theory, change the facts. Do please keep things simple, do produce an explanation without the cool atmosphere making the surface hotter than the atmosphere itself. (Hint, try the effects of gravity and adiabatic heating, or is that not allowed in "climatology"?)--Damorbel (talk) 17:03, 20 March 2010 (UTC)
- What on earth does any of this have to do with cosmic rays? Yes, clouds are a part of the greenhouse effect (although they change the earth's albedo as well, so it's a little complicated), so is water vapour, so is methane, so is ozone, so are CFCs. This is explicitly mentioned in the article, and this article in particular doesn't pretend that the greenhouse effect is due to one thing and one thing alone.
- I think it's worth considering that even if it is clouds responsible for the greenhouse effect, they are still violating your imaginary second law of thermodynamics. While you acquaint yourself with the actual theory, I suggest you also review the Dunning–Kruger effect to ensure you haven't become a victim of that as well. The suggestion that every physicist in the world has missed the fact that the entire field of climatology violates one of the most fundamental physical laws is ludicrous. StuartH (talk) 20:36, 20 March 2010 (UTC)
- Well, actually you are the first one to describe the second law of thermodynamics in such a special way, i.e. applying it to EM radiation moving in a unidirectional way (warm to cool), while refusing to use the concept of a net flow. I notice you chose not to address any of the numbers in the chart. I notice that your link to perpetual motion is indeed curious since your response to my challenge some time back led to the creation of a perpetual motion machine. As for your use of the term "body" I'll initiate another play-dumb game. When you used the term "body" did you mean a large mass (in particular large enough that common ways of measuring temperature can be used)? Do you also apply the term to single atoms/molecules? (Do you see the beginnings of the trap?) And, yes, I fixed your indents, again. blackcloak (talk) 19:50, 20 March 2010 (UTC)
- Blackcloak. You say "actually you are the first one to describe the second law of thermodynamics in such a special way" Heat is transferred with radiation from hotter to cooler by the same law, even the GH Effect seems to recognise this. Haven't you just written something rather foolish? --Damorbel (talk) 13:32, 21 March 2010 (UTC)
- I guess we have to revert to basics. Do you believe in the concept of negative numbers? Assuming you answer yes, do you believe that if you add a negative number to a positive number that the result of the summation is a number that is less than the positive number? If I get the correct answer the these two questions, I'll know I can move on to a discussion of "net" flow. blackcloak (talk) 04:16, 22 March 2010 (UTC)
- There's a good post on that flawed second law of thermodynamics here: [4]. You are arguing using an imaginary law of thermodynamics that physicists do not use. You clearly do not understand the laws of thermodynamics, so it would be a good idea to check that page out. StuartH (talk) 20:01, 20 March 2010 (UTC)
- StuartH "What on earth does any of this have to do with cosmic rays?" I suggest you wise up on Svensmark's work. For clouds to form from water vapour cloud condensation nuclei are required, Svensmark's hypothesis is that these arise from cosmic rays [5] thus the presence of clouds (with or without precipitation) is related to the level of cosmic rays passing through the atmosphere. Clouds certainly influence heat transfer in the atmosphere by absorption and and emission but they also reflect radiation which CO2 does not. Reflected radiation, with the reflector configured suitably, can lead to its trapping or exclusion, this is how a vacuum flask works. This trapping effect is one way that "warm cloudy nights" can be explained. Also, by absorbing the Sun's radiation, clouds suppress convection at ground level, transferring it to the cloud top.
- StuartH "There's a good post on that flawed second law of thermodynamics here: [6]."The 2nd Law deals only with temperature difference and energy transfer, the energy transfer showing up only as a change in temperature. To quote your link "My boring thermodynamics books and I have long since had a parting of the ways" Where the author goes wrong (as does the greenhouse effect article, see this diagram [7]) is assigning Watts i.e. energy, to a radiation field; how ever many watts are in the radiation field there is no "net" (i.e. heat) energy transfer by the radiation field unless there is also a temperature difference. A red hot mass of iron is generating radiation from every atom but heat is transferred only from high temperature parts to low temperature parts. Now for iron, as a solid, heat is tranferred by vibrations as well as radiation. In a gas heat is trasnsferred by atomic (or molecular) collisions, see kinetic theory as well as radiation for the so called GH gases, there is nothing special about this but it does explain why some gases glow in the infrared when hot and others don't e.g. helium, argon etc. (N.B. these gases do glow when ionised, this does not happen at the Earth's atmospheric temperatures.)--Damorbel (talk) 13:32, 21 March 2010 (UTC)
- You're still not conveying a clear understanding of thermodynamics at all. Firstly, Svensmark's speculative hypothesis about the origins of clouds is irrelevant to the question of whether or not clouds violate the second law of thermodynamics. "It's reflection" isn't an answer either, you cannot violate the second law using reflection, and a vacuum flask at no point allows the net flow of heat from cooler to warmer. It inhibits the rate of flow from warm to cool, exactly how the greenhouse effect works.
- The diagram doesn't just include the transfer of heat through radiation, it includes all heat flows between the surface and the atmosphere. To demonstrate a violation of the second law, you need to demonstrate where there is a net flow of heat from a cooler body to a warmer body. There are four bodies of interest here -- the sun's surface, the earth's surface, the atmosphere and free space. Every possible combination of these involves a flow of heat from warmer to cooler. It is also assumed in the simplified explanation that the earth's surface is in balance, and so is the atmosphere -- that is, heat in equals heat out and there is no warming over time. Because the greenhouse effect is changing and the planet is warming, this isn't actually true, but it's still a useful explanation. So where is the net flow of heat from cooler to warmer? StuartH (talk) 16:46, 21 March 2010 (UTC)
- StuartH "It inhibits the rate of flow from warm to cool, exactly how the greenhouse effect works." Read the Greenhouse effect article carefully, the effect attributed to CO2 is that "it radiates to the surface warming it" look at the diagram [8] and read what it says at http://en.wikipedia.org/wiki/Greenhouse_effect#Basic_mechanism. The basic mechanism is about as clear as mud but it is supposed to be radiation from CO2, H2O etc. that raises the temperature of the surface by 33K above its equilibrium temperature of 255K. "So where is the net flow of heat from cooler to warmer?" Good question, but the surface will only have its temperature raised above the equilibrium by radiation from CO2 if there is a a net inflow of energy and that cannot come from the co\d troposphere for the reasons I gave. --Damorbel (talk) 14:13, 22 March 2010 (UTC)
- Yes, that is how greenhouse gases inhibit the flow of heat from the surface to free space -- by absorbing some of the radiation and reradiating it to the surface. If it's clear as mud to you and you have a suggestion to improve it, voice that suggestion by all means, that is what the talk page is supposed to be about after all. If you're unsure how the surface of the earth can heat up, take another look at the graph. The surface gets 168 W/m² from the sun, 324 W/m² from the atmosphere, loses 452 W/m² to the atmosphere, and loses 40 W/m² to empty space. Energy in equals energy out. If you enhance the greenhouse effect, less radiation escapes into free space and more is returned to the surface. The surface will then heat up until radiative balance is restored. The net flow between the surface and the atmosphere remains positive, and at no point violates the second law. The surface is warming because the flow from the sun remains the same while the net flow into the atmosphere goes down. StuartH (talk) 23:39, 22 March 2010 (UTC)
break 1
- Doesn't "net flow into the atmosphere goes down" mean that the atmosphere will get cooler? Q Science (talk) 04:44, 23 March 2010 (UTC)
- Not if the atmosphere is also radiating less heat out into free space. In fact, the atmosphere warms because the net flow out into free space falls by more than the net flow into the atmosphere from the surface. StuartH (talk) 05:23, 23 March 2010 (UTC)
- So, if you increase the number of emitters, then the amount of radiation to space decreases? Also, when the atmosphere's temperature increases, the radiation to space decreases? Q Science (talk) 07:05, 23 March 2010 (UTC)
- I'm not quite sure what you mean here... when you say "increase the number of emitters" do you mean increase it from just the surface to the surface + the atmosphere? It's not a case of adding independent emitters. What's happening is that adding greenhouse gases absorbs radiation that would otherwise make it through the atmosphere unimpeded. Some of it gets re-radiated to the surface, some of it gets re-radiated out into space (but less effectively because the atmosphere is cooler than the surface). When you say "when the atmosphere's temperature increases, the radiation to space decreases", I think you're looking at it backwards. The greenhouse effect traps heat and decreases the loss of heat from the atmosphere through radiation. That warms the atmosphere (and surface, indirectly)... so that is the link between falling radiation to space and a heating of the atmosphere. But as the surface and atmosphere warm, they in turn emit more radiation and eventually a warmer equilibrium is reached. I hope some of this is making sense... StuartH (talk) 09:47, 23 March 2010 (UTC)
(outdent) I think we disagree on the following
- The greenhouse effect traps heat and decreases the loss of heat from the atmosphere through radiation.
It should say
- The greenhouse effect traps heat and decreases the loss of heat from the surface directly to space through radiation.
In fact, the theory specifically says that the radiation from the surface will increase if CO2 is increased, but that a smaller amount will be able to reach space. Q Science (talk) 06:06, 26 March 2010 (UTC)
- Ah, good point. I should probably have said "The surface is warming because the flow from the sun remains the same while the total net flow from the surface decreases". On the other hand, only 40 W/m² escapes directly into space, with most of it actually radiated from the atmosphere. The greenhouse effect doesn't just apply to the surface, but to radiation from the lower, warmer atmosphere. So I think both are actually correct -- loss from the atmosphere to space and loss from the surface to space both go down, but I'm not 100% sure of what happens to the net flow from the surface to the atmosphere now. It definitely remains positive, but whether it increases or decreases depends on whether reradiation down to the surface exceeds the absorption of surface radiation that would otherwise make it straight out into space. This is drifting a little from my area of expertise, maybe a real climatologist could clarify. I'm guessing it depends on the absorption spectrum and the current concentration of greenhouse gases. StuartH (talk) 08:30, 26 March 2010 (UTC)
- The amount of energy from the Earth to space can not change, it must be equal to the amount received from the Sun. Therefore, it is not possible for the amount released from the surface AND the amount released from the atmosphere to both decrease unless the albedo increases. Since warming causes ice to melt, it is generally thought that the albedo will decrease. Q Science (talk) 15:56, 26 March 2010 (UTC)
- You're talking about the equilibrium, I've been talking about the change before equilibrium is reached. Initially, heat loss from the earth goes down, until the earth warms and the heat loss eventually matches the heat gain from the sun. At equilibrium, a stronger greenhouse effect certainly implies a decrease in radiation directly from the surface and a corresponding increase from the atmosphere, but if you take "the amount of energy from the Earth to space cannot change" too far, it would be impossible for the greenhouse effect to warm the earth. The amount of energy radiated out into space can decrease, but that warms the earth until it increases back to the equilibrium position. StuartH (talk) 23:10, 26 March 2010 (UTC)
- Don't use the word trap because the idea of releasing the heat is not included. If you insist on using trap then also, for each time trap is used, also use release. Heat moves into the atmosphere during the day and out of the atmosphere during the night. I prefer the words "temporarily retain" to describe the process. An equilibrium point is somewhere between the minimum and maximum points in the diurnal retained heat profile. We use this magic equilibrium point (estimate) for the purpose of computing long term thermal balance numbers. Long term, energy in equals energy out (except for the small amount that might be attributed to global warming, say .05 degrees per year, and a variation well in the noise of temperature estimates in any given year). Short term (hours), the is no equilibrium, and energy in from the Sun does not equal energy out (IR out into space, plus reflected sunlight). blackcloak (talk) 20:15, 26 March 2010 (UTC)
- True, I guess some care does need to be taken with the terminology used. "Trap" is a simplification that isn't entirely accurate. StuartH (talk) 23:10, 26 March 2010 (UTC)
outdent and break
Think of a laser pointer. It does not matter what you point it at, the number of photons per second remains the same. When you point it at the Sun, the number of photons is the same as when you point it at a wall. Blackbodies (and greenhouse gases) are the same, they emit a certain amount of energy (as photons) depending on their temperature. It does not matter if nearby objects are hotter or colder, the emitted watts per square meter are the same. This is how a cold atmosphere is able to help heat a warmer body. In the case of the atmosphere, energy from a 270K atmosphere raises the temperature of the Earth by about 33K. Notice, the atmosphere does not heat the surface to 288K, but it adds 33K to the temperature produced by the Sun. Q Science (talk) 16:53, 21 March 2010 (UTC)
- I have to disagree. A laser is not a black body, it is monochromatic, it output doesn't conform to a blackbody spectrum. The amplitude of its output (at its characteristic frequency) could be that of any number of blackbody temperatures. But a blackbody emits energy on a vast number of frequencies, to be a true black body the spectrum must be the same as that defined by the Planck formula, the Planck spectrum. Even so the laser will transmit energy to a hot body if the irradiation from the laser has a greater amplitude than the amplitude of the blackbody spectrum at that wavelength (and vice-versa), it is just that the word temperature has lost its meaning.
- With the last few words of your entry, you're starting to get closer. EM radiation interacts with individual atoms/molecules, therefore affecting the energy of a picoscopic, local volume. The concept of temperature at such a microscopic level becomes hard to define. I could attempt to provide one, but it would be quite a diversion from the thread of this discussion. It is easier to restrict oneself to a macroscopic definition of temperature, and perhaps further restrict any discussion of temperature change to relatively large changes in a (macroscopic) body's temperature like 0.000001 degrees- i.e. the change in temerature of say a cubic mm of water after absorbing say 10^10 IR photons (guessing on orders of magnitude). blackcloak (talk) 04:33, 22 March 2010 (UTC)
- If you consider two adjacent blackbodies (or two blackbodies with their images focussed on each other by a lens) the temperatures are not added, don't you think that would be absurd? They just exchange energy according to the temperature difference, that is how heat works.--84.196.128.194 (talk) 22:09, 21 March 2010 (UTC)
- If a laser bothers you, then consider a flash light. (Hot wires are blackbodies.) It really does not matter. The point is that anything that emits photons will do so and it does not matter if the photons are traveling toward a dark or bright object or to a hot or cold object. Q Science (talk) 01:24, 22 March 2010 (UTC)
- Q Science, a laser should bother you for the reason I give i.e the spectrum of a laser has nothing to do with blackbody radiation and thus temperature which is at the heart of the GHE hypothesis.
- Take your flashlight, let us say the filament is at 2700K, to avoid getting your fingers burnt you focus its image on the Sun at 5780K but don't forget that simultaneously you have also focused the Sun's image on the filament, melting point 3695K, what happens?.--Damorbel (talk) 08:08, 22 March 2010 (UTC)
- Because of 1/R2, the effective temperature of the sun at the top of the atmosphere is only 120°C (394°K), somewhat less at the surface. So, the filament is much hotter than the Sun. The reason it is dimmer has to do with the total number of photons emitted which is dependent on temperature AND the surface area. However, your focusing argument is good. There are solar furnaces where an array of mirrors focus the Sun to a small spot, reaching temperatures above 3,500°C. These work because IR radiation is additive which, it turns out, is also why cold Greenhouse gases are able to heat the surface. To paraphrase a bit, the equilibrium temperature of an object is based on the sum of the radiation from all available sources. You can prove that this is true by starting a fire with a magnifying glass. Q Science (talk) 17:09, 22 March 2010 (UTC)
- "total number of photons emitted which is dependent on temperature" No Q Science, the number of photons is not dependent on the temperature it is the energy of the photons that is dependent on the temperature, see Wien's displacement law, that is why I inserted a lens, the maximum temperature that can be achieved in a focused image of an incandescent source depends only on the source temperature. The number total number of photons coming from a source depends on the number of emitting particles, which may indeed be related to the area. --Damorbel (talk) 21:18, 22 March 2010 (UTC)
- This distinction between number an energy of photons is the reason why the climatological energy balance diagrams with Watts per square metre all over the place are really not up to the job of describing the themal behaviour of the planetary atmosphere. For heat transfer it is neccessary to know the energy of the photons because 1W/m^2 may comprise a large number of low energy photons (from a low temperature source) or a fewer number of higher energy photons (from a source with a higher temperature). --Damorbel (talk) 21:18, 22 March 2010 (UTC)
- When you increase the temperature of a blackbody, the color changes (Wien's displacement law) and it gets brighter (number of photons increases, see the first image in Blackbody). Q Science (talk) 04:58, 23 March 2010 (UTC)
- Photons are emitted by particles, one at a time. The hotter the particles the higher the photon energy, basic quantum theory. The energy of the photons is the product of the characteristic frequency and Planck's constant E=hv.
- This description of an oversimplified understanding fails to express the true nature of, ahem, nature. The temperature of a "particle" determines an associated bb curve, the peak of the emission (power vs. wavelength) curve occurring at a wavelength that depends on that temperature. A large portion (depending on where you set thresholds) of the emitted radiation is well above (longer in wavelength) the "peak wavelength" and some is well below the "peak wavelength." Bringing E=hv into the discussion is a red herring since the equation gets at temperature indirectly through energy. This is classic misdirection, one of Damorbel's favorite passtimes. If he wanted to make his point based on physics, he would provide a basic equation that expresses a functional dependence of emitted photon frequency on "particle" (presumably, macroscopic) temperature. And, taking a bit of a stretch here, I'll assert such an equation does not exist. blackcloak (talk) 16:54, 23 March 2010 (UTC)
- Blackcloak, I don't think your contribution adds anything to my necessarily simplified explanation of why photon energy plays a vital roll not only in quantum interactions but in heat transfer also. After all, Planck's law of thermal radiation was the first accurate description of what was later described as quantum mechanics.--Damorbel (talk) 19:52, 23 March 2010 (UTC)
- What morsel survives contempt when seeming wisdom defiles logic? blackcloak (talk) 07:30, 24 March 2010 (UTC)
- Blackcloak, I don't think your contribution adds anything to my necessarily simplified explanation of why photon energy plays a vital roll not only in quantum interactions but in heat transfer also. After all, Planck's law of thermal radiation was the first accurate description of what was later described as quantum mechanics.--Damorbel (talk) 19:52, 23 March 2010 (UTC)
- This description of an oversimplified understanding fails to express the true nature of, ahem, nature. The temperature of a "particle" determines an associated bb curve, the peak of the emission (power vs. wavelength) curve occurring at a wavelength that depends on that temperature. A large portion (depending on where you set thresholds) of the emitted radiation is well above (longer in wavelength) the "peak wavelength" and some is well below the "peak wavelength." Bringing E=hv into the discussion is a red herring since the equation gets at temperature indirectly through energy. This is classic misdirection, one of Damorbel's favorite passtimes. If he wanted to make his point based on physics, he would provide a basic equation that expresses a functional dependence of emitted photon frequency on "particle" (presumably, macroscopic) temperature. And, taking a bit of a stretch here, I'll assert such an equation does not exist. blackcloak (talk) 16:54, 23 March 2010 (UTC)
- I have tried to get the concept of low energy and high energy photons accross. Try looking at it this way, it is exactly the same as with matter; matter contains energy proportional to its temperature, a large amount of matter at a low temperature may very well contain exactly the same energy as a smaller amount at a higher temperature, the important fact being the higher temperature material may burn your fingers whereas the larger, cooler mass won't.--Damorbel (talk) 08:26, 23 March 2010 (UTC)
- Photons are emitted by particles, one at a time. The hotter the particles the higher the photon energy, basic quantum theory. The energy of the photons is the product of the characteristic frequency and Planck's constant E=hv.
- I think the number of photons is pretty much irrelevant, but the simplified diagram is just representing the heat flux between the surface, sun, atmosphere and free space. In this context it doesn't matter how much energy each photon has -- i.e. what the wavelength of the photons is, it's a straight heat balance diagram. Obviously the diagram isn't going to describe every aspect of the climate completely, but it doesn't attempt to. If you have what you believe to be a better diagram, suggest it for inclusion. StuartH (talk) 05:47, 23 March 2010 (UTC)
- "what you believe to be a better diagram" You cannot consider heat flow without accounting for temperature differences (very basic science!) no tempperature difference no - heat flow. The whole concept of the heat flow diagrms showing W/m^2 in the Greehouse effect is mistaken. It may be difficult to accept but when you think about it temperature difference is what drives heat flow, the very idea that heat (that is heat energy) can flow somehow from the colder tropsphere to the warmer surface, causing warming of the surface, is a classic misunderstanding of thermodynamics --Damorbel (talk) 08:26, 23 March 2010 (UTC)
- There is no net flow from the cool atmosphere to the warm surface. You have the numbers from the graph, and it would be trivial to demonstrate a violation of the second law if it were there. If you have no further interest in improving the article or understanding the basic physics, I have no further interest in continuing this discussion. I can only suggest that you consider how unlikely it is that the entire field of climatology violates one of the most fundamental physical laws, while every physicist in the world allows them to do so. If it's between you being wrong and every physicist and climatologist in the world being wrong... maybe it's you. StuartH (talk) 09:24, 23 March 2010 (UTC)
- No one is saying what you're attributing to others. Here is what they are saying, using your words and terms: ..heat can flow from a cooler body to a warmer body, causing the warmer body to cool more slowly than it would were it not for the presence of the cooler body. I know that may sound unnecessarily complicated to you, but that fairly describes the complexity required to explain nature. blackcloak (talk) 01:23, 24 March 2010 (UTC)
- Blackcloak "..heat can flow from a cooler body to a warmer body, causing the warmer body to cool more slowly" this not what the GHE claims, what you describe here is conduction of heat which is a diffusion process governed by the heat equation. Your remark "to cool more slowly" indicates your line of thinking since the diffusion equation governs changes of heat distribution with time. I recommend the heat conduction article since it emphasises the distinction between heat conduction, convection and radiation.--Damorbel (talk) 09:11, 24 March 2010 (UTC)
- No. I was using your words and terms to describe the effect of net energy flow via em radiation, the very case (I thought) you (thought you) were describing. blackcloak (talk) 18:51, 24 March 2010 (UTC)
- Blackcloak "..heat can flow from a cooler body to a warmer body, causing the warmer body to cool more slowly" this not what the GHE claims, what you describe here is conduction of heat which is a diffusion process governed by the heat equation. Your remark "to cool more slowly" indicates your line of thinking since the diffusion equation governs changes of heat distribution with time. I recommend the heat conduction article since it emphasises the distinction between heat conduction, convection and radiation.--Damorbel (talk) 09:11, 24 March 2010 (UTC)
- No one is saying what you're attributing to others. Here is what they are saying, using your words and terms: ..heat can flow from a cooler body to a warmer body, causing the warmer body to cool more slowly than it would were it not for the presence of the cooler body. I know that may sound unnecessarily complicated to you, but that fairly describes the complexity required to explain nature. blackcloak (talk) 01:23, 24 March 2010 (UTC)
- There is no net flow from the cool atmosphere to the warm surface. You have the numbers from the graph, and it would be trivial to demonstrate a violation of the second law if it were there. If you have no further interest in improving the article or understanding the basic physics, I have no further interest in continuing this discussion. I can only suggest that you consider how unlikely it is that the entire field of climatology violates one of the most fundamental physical laws, while every physicist in the world allows them to do so. If it's between you being wrong and every physicist and climatologist in the world being wrong... maybe it's you. StuartH (talk) 09:24, 23 March 2010 (UTC)
- "There is no net flow from the cool atmosphere to the warm surface." I agree. But the GHE article maintains that the surface is heated 33K above the equilibrium temperature by radiation from GH gases, how does this happen? --Damorbel (talk) 09:34, 23 March 2010 (UTC)
- The article does a reasonable job of explaining this. Without a greenhouse effect, the earth would equilibrate at a lower temperature because it can radiate the sun's 235 W/m² directly into space. With a greenhouse effect, equilibrium is reached at a higher temperature because much of the heat radiated from the surface stays in the atmosphere/surface system. It warms up until emitted radiation rises enough to compensate. StuartH (talk) 10:21, 23 March 2010 (UTC)
- "what you believe to be a better diagram" You cannot consider heat flow without accounting for temperature differences (very basic science!) no tempperature difference no - heat flow. The whole concept of the heat flow diagrms showing W/m^2 in the Greehouse effect is mistaken. It may be difficult to accept but when you think about it temperature difference is what drives heat flow, the very idea that heat (that is heat energy) can flow somehow from the colder tropsphere to the warmer surface, causing warming of the surface, is a classic misunderstanding of thermodynamics --Damorbel (talk) 08:26, 23 March 2010 (UTC)
- StuartH "The article does a reasonable job of explaining this". Since the effect of carbon dioxide is said to be that the surface is warmed by radiation from CO2 in the troposphere, it is very difficult to ignore the fact that the surface of the Earth is always warmer than the troposphere above, thus the explanation ignores the basic facts of heat transfer, I fail completely to see how this does "a reasonable job."--Damorbel (talk) 19:52, 23 March 2010 (UTC)
- It is fundamental for thermal equilibrium that the planet radiate away all 235 W/m² that it has absorbed. Because of the greenhouse effect, the only way the top of the atmosphere can radiate this much is if, beneath it, there is an even higher temperature providing enough net inflow into the bottom of the atmosphere that the atmosphere can radiate a lot of that back down again and still have enough heat left to put 235 W/m² back out upwards into space. When a person sleeps under a blanket, the blanket is colder than their body, but still keeps them warmer than if it wasn't there. The difference then is that the unchangeable fact is that the person's body will have to lose, I dunno, 300 W all night and no one cares what escapes the blanket, so the equilibrium is caused by a different thing, but still it's warmer under the blanket. Wouldn't a better place for these questions be Wikipedia:Reference desk, as they must be helping people understand the physics, but they are not helping to improve this article per WP:TPG. --Nigelj (talk) 10:35, 23 March 2010 (UTC)
- Nigelj, are you seriously comparing the Earth's atmosphere to a blanket?. One tends to think you are joking, funny for today. Wikipedia suffers quite a lot from such contributions, they waste space and are only sometimes funny. There is nothing in the GHE article to suggest a blanket. --Damorbel (talk) 15:25, 23 March 2010 (UTC)
- I think you need to have a look at WP:TPG and decide in which way you would like to help improve this article, then make your suggestions here if you have any. Then have a look at WP:NPA and bear in mind that this article and this talk page are under article probation. All of this is explained at the top of this page. --Nigelj (talk) 16:12, 23 March 2010 (UTC)
- I suggest that you should first explain the blanket idea here if it is to be included in the article. I would very much like to have a clear idea what particular charateristics of a blanket are to be considered relevant to an atmosphere? Also, are there any limitations in the analogy? That is to say are some features of a blanket to be excluded from the atmosphere as a blanket model? --Damorbel (talk) 16:29, 23 March 2010 (UTC)
- My answer to your question above was given in the spirit of Wikipedia:Reference desk, but any further such questions should, as I said, be directed there. Well-sourced suggestions to improve this article are, of course, welcome here. Thank you for your interest. --Nigelj (talk) 16:49, 23 March 2010 (UTC)
- Nigelj, I think I understand from this that you consider explanations involving the atmosphere behaving like a blanket are not appropriate in an article about the Greenhouse effect.--Damorbel (talk) 19:52, 23 March 2010 (UTC)
- It is time to close this. Wikipedia is not a general forum for discussion (even about the topic of the article). Damorbel if you have griefs about the greenhouse effect and the laws of thermodynamics then you can take it to the Wikipedia:Reference desk. So far you have only been a time-sink. I'm sorry to have to inform you, that your interpretation is wrong - and we are not here to correct you. --Kim D. Petersen (talk) 20:31, 23 March 2010 (UTC)
- I'm starting to agree. I made a good faith attempt to correct a clear misunderstanding of another contributor while maintaining a sliver of hope that the discussion could improve the article. Explanations for Damorbel's misunderstanding have been presented by more knowledgeable people, and Damorbel either willingly ignores those explanations or lacks the capacity to understand them. I have no further interest in contributing to this discussion without the possibility of improving the article. StuartH (talk) 21:23, 23 March 2010 (UTC)
- It is time to close this. Wikipedia is not a general forum for discussion (even about the topic of the article). Damorbel if you have griefs about the greenhouse effect and the laws of thermodynamics then you can take it to the Wikipedia:Reference desk. So far you have only been a time-sink. I'm sorry to have to inform you, that your interpretation is wrong - and we are not here to correct you. --Kim D. Petersen (talk) 20:31, 23 March 2010 (UTC)
- Nigelj, are you seriously comparing the Earth's atmosphere to a blanket?. One tends to think you are joking, funny for today. Wikipedia suffers quite a lot from such contributions, they waste space and are only sometimes funny. There is nothing in the GHE article to suggest a blanket. --Damorbel (talk) 15:25, 23 March 2010 (UTC)
- Ah! KimDabelsteinPetersen, "I'm sorry to have to inform you, that your interpretation is wrong - and we are not here to correct you." So kind, your clear explanation of the physics and a firm decision!--Damorbel (talk) 21:51, 23 March 2010 (UTC)
- This matter has been explained to you, again and again, over years, at all levels between expert and kindergarten. Either you are incapable of getting it, or you are a troll. Please stop wasting our time. Thanks. --Stephan Schulz (talk) 07:59, 24 March 2010 (UTC)
- Stephan, you write 'This matter has been explained to you, again and again'. I understand from this you are citing the arguments of others, not your own. This suggests you are unable to make the case yourself for CO2 warming the surface by as described in the GHE article. Now the article claims that CO2 (and other GHGs) in the atmosphere are transfering heat to the surface by radiation and warming said surface by 33C above its equlibrium temperature; this is also the position of the IPCC. The argument is deeply flawed in that atmospheric CO2 is always colder than the surface; what actually happens is the heat flows from the surface towards the GHGs, i.e. the direct opposite of the claim. The relevant science of heat transfer is called (thermodynamics) has been established for many years. One of its most tested laws is the 2nd law which says it is impossible for heat to flow from a cold body to a hotter one, it always flows in the other direction, warming the colder, never the hotter; as happens in common experience.
- Now it may well be that you are able to make the case for surface warming by tropospheric gases, personally I cannot wait to read it because, should it be a good one, the world of physics would have to be rebuilt from its very foundations--Damorbel (talk) 19:07, 24 March 2010 (UTC)
- Apparently your memory is failing. I explained this stuff to you ages ago, for about an eternity. Unfortunately, you have learned nothing from it. George Santayana had something appropriate to say about this... --Stephan Schulz (talk) 22:16, 25 March 2010 (UTC)
- OK, skip the reference desk. Write direct to the IPCC, tell them they got it wrong, then come back here with the new reference when they publish their correction based on your research. Until then there's nothing we can do for you here. --Nigelj (talk) 20:51, 24 March 2010 (UTC)
- Nigelj, "Write direct to the IPCC, tell them they got it wrong". Poor IPCC! Yet again? What with Pachauri's melting glaciers and Mann's straight hockey stick, surely there is enough to ensure its oblivion.--Damorbel (talk) 22:07, 25 March 2010 (UTC)
- If you actually read 2nd lawyou'd know that it is not impossible for heat to move from a cooler particle to a warmer particle. Experiments have confirmed that this can occur. You'd also know that the law is intended to apply only to macroscopic systems. An emitting molecule and an absorbing molecule do not constitute a macroscopic system. And, you might even note that radiative heat transfer is not even discussed. In fairness to us, at the very least, your arguments should not be contradicted by your own sources. blackcloak (talk) 02:12, 25 March 2010 (UTC)
- Blackcloak, "Experiments have confirmed..." I think this statement deserves at least two links, and not to a refrigerator... please!--Damorbel (talk) 21:52, 25 March 2010 (UTC)
- I respectfully decline your request for what should be obvious reasons. blackcloak (talk) 07:20, 2 April 2010 (UTC)
- Blackcloak, "Experiments have confirmed..." I think this statement deserves at least two links, and not to a refrigerator... please!--Damorbel (talk) 21:52, 25 March 2010 (UTC)
- This matter has been explained to you, again and again, over years, at all levels between expert and kindergarten. Either you are incapable of getting it, or you are a troll. Please stop wasting our time. Thanks. --Stephan Schulz (talk) 07:59, 24 March 2010 (UTC)
- It is fundamental for thermal equilibrium that the planet radiate away all 235 W/m² that it has absorbed. Because of the greenhouse effect, the only way the top of the atmosphere can radiate this much is if, beneath it, there is an even higher temperature providing enough net inflow into the bottom of the atmosphere that the atmosphere can radiate a lot of that back down again and still have enough heat left to put 235 W/m² back out upwards into space. When a person sleeps under a blanket, the blanket is colder than their body, but still keeps them warmer than if it wasn't there. The difference then is that the unchangeable fact is that the person's body will have to lose, I dunno, 300 W all night and no one cares what escapes the blanket, so the equilibrium is caused by a different thing, but still it's warmer under the blanket. Wouldn't a better place for these questions be Wikipedia:Reference desk, as they must be helping people understand the physics, but they are not helping to improve this article per WP:TPG. --Nigelj (talk) 10:35, 23 March 2010 (UTC)
Rule 5, to the fifth power, five times every five days for five years. Short Brigade Harvester Boris (talk) 01:32, 27 March 2010 (UTC)
Proof?
There is disputed proof on the Greenhouse effect existing. Carbon is a natural element of planetary life, on top of that the Greenhouse effect has shown significant water level rise before the 20th ce. I have two maps of India, one map is 600 years old. The shoreline in the old map shows that most of the google earth villages and cities around the shoreline right now was underwater. I also study geology so I was able to simulate a map of the ice age where most of the continent, through drift analysis, was underwater 80,000 years ago. The continent of India looked much like a sharp narrow tooth. So to say water is receding or climbing due to climate change is a lie. It can recede and it can expand because of a minute geological shift, or when plates move quickly over a given time by planetary impact (this happens when most life is extinct). When the earth turns everything moves quickly. The state of life is then returned to savagery which later through time does chain reactions of the environment occur. The water or enormous current then decides which land sinks and which doesnt. Eg sea level rise. The melting of the iceage's glaciers was proof of this fundamental law of sea levels rising and still is occuring today through natural process. The lowering of sea levels can be because of geogical shifts and occurs naturally in low level seas or old ocean areas. The idea about marketing is interesting. It holds fundamental laws to some shoreline areas to be true, albeit the facts are not verifiable. Eg over time Mars will die like any planet, its carbonated gas will be on the planet's surface like the dead life of organisms is in the oil of our planet. It will collect over a large period of time. --64.9.241.30 (talk) 00:45, 30 March 2010 (UTC)
- ...and your suggestion for improving this article is...?--CurtisSwain (talk) 11:53, 2 April 2010 (UTC)
Not keen
On [9]. Calling outer space a thermal reservoir isn't right William M. Connolley (talk) 16:48, 14 April 2010 (UTC)
- Good point, I've rephrased it slightly. The caption is getting a bit long anyway... StuartH (talk) 06:20, 15 April 2010 (UTC)
- blackcloak here. Having logon/pw problems that probably will mean a change in my id. Very disturbing how little fault tolerance is built into this system. Anyway, I'm not married to the use of the words "thermal reservoir." I considered "thermal sources/sinks" but rejected it as overly complex for the context. "thermal reservoir" is not that bad, in my opinion. "outer space" has lots of 3 degree Kelvin volume (finite temperature and finite density, mostly a sink) and occasional high temperature hot spots (stars, sources). Certainly isn't homogeneous, but like more common thermal reservoirs, energy moves in and out of it. Of course, you haven't explained why you think the term isn't right. I might be barking up the wrong tree. blackcloak








