Talk:Black body
| Black body was nominated as a good article, but it did not meet the good article criteria at the time (July 21, 2006). There are suggestions below for improving the article. If you can improve it, please do; it may then be renominated. |
| This article is of interest to the following WikiProjects: | |||||||||||||||||||||||||||
Please add the quality rating to the {{WikiProject banner shell}} template instead of this project banner. See WP:PIQA for details.
Please add the quality rating to the {{WikiProject banner shell}} template instead of this project banner. See WP:PIQA for details.
| |||||||||||||||||||||||||||
|
Good Article nomination has failed
The Good article nomination for Black body has failed, for the following reason:
- (This article needs a lot more sources. The entire Explanation section needs citation. I'm instead nominating this article for Unreferenced Good Article status) Talkstosocks 03:44, 22 July 2006 (UTC)
Radiation Emitted by a Human
In the summary of my 02:55, 9 May 2006 (UTC), I misunderstood the previous version of JabberWok, where the surface temperature was reduced by clothing. If the surface is the surface of the clothes, then it is true. However, I still think that it is too complicated to explain here and we should not change temperatures to something guessed. Poszwa
- Yes I was talking about the surface temperature of the clothes so that they'll cause you to emit less radiation to the environment overall. I don't like the current value of 164 Watts as that bring the minimum amount of food one would have to eat up to that incredible amount of 3,400 calories. Lets find a more reasonable way to estimate how much electromagnetic energy a person emits in a day. I guess I could walk down the hall to our supply room and put a thermometer on my shirt if you don't like my guessed value.JabberWok 21:01, 9 May 2006 (UTC)
- It's 160 Watts because you would start to tremble increasing your heat production at the moment. I know it is hard to do it all day long and this number looks strange but with those assumptions it was right. I didn't want to guess any "more corrent" numbers and I didn't find any numbers to cite. Poszwa 00:29, 10 May 2006 (UTC)
Ok, so I know Wikipedia isn't the place for original research, but just out of curiosity I had to see for my self....So I went and grabbed a Fluke 51 thermometer out of the supply closet to find my average surface temperature. My exposed skin - face and arms - have an average temp of about 30.5 C, and my clothes - t-shirt and pants - have an average temp of 25.8 C. And this is in a room with a temp of 20.2 C.
So, can we just go with human surface temp of about 28 C (301 K) and a room temp of about 20 C (293 K)? This results in a person being something like 95 Watts. (We could round and just say 100 Watts.)
And emitting 100 watts for 24 hours turns out to be...2,065 calories of energy! JabberWok 22:40, 9 May 2006 (UTC)
- Your measured temperatures are OK but people normally don't radiate 100 Watts because part of the heat is transfered by other means and according to the link presented at the end of the article [1], it is a few tens of Watts. Showing an example that suggests that all of the energy consumption is radiated can be misleading. If we put 26-27 C and 20C ambient, we would get 71-83 W, which is probably more reasonable. Or we can use the example from the above-mentioned link, where they assume 34C skin and 23C ambient (but then the radiation is 130 W). Anyway, if the temperature is changed, the peak wavelength calculated below should also be changed. Poszwa 00:29, 10 May 2006 (UTC)
- The article is misleading when it says that 2000 kcal of energy are spent on radiative cooling (for a typical 40 year old adult male). This may be true (up to the rough estimates), but it ignores that as a black body, humans also absorb radiation and thus are heated. So to look at the "net" loss due to radiative cooling, in addition to the radiative loss from skin/clothing at about 30 C, you should add in radiative heating from ambient surroundings at roughly 20 C (using the same surface area). 131.215.143.20 (talk) 19:56, 15 February 2010 (UTC)
≈ 1!
The emissivity of human skin is apparently .98 [2], so it is a close approximations of a black body. Does the .02 less of 1 constitute that radiation which is reflected off of a skin sample that I then see as its color? --HantaVirus 15:55, 27 July 2006 (UTC)
The analysis in this section of the article explicitly neglects convective heat losses, attributing essentially all the metabolic heat loss to radiation. While the calculation using an 8 degree temperature difference gives heat loss roughly equal to resting metabolic rate (RMR), there is plenty of wiggle room in the assumed values. Most body surface area is likely to be covered by clothing or hair in an 20 C environment for a resting individual to be comfortable. Hence, a number closer to 6 degrees is probably justified. Maybe it is even lower. Perhaps the emissivity of clothing is significantly less than 1.
On the other hand, the convective coefficient can be estimated to be on the order of 10 W/m^2/K. One such measurement is for a flat, vertical plate that gives 10 (http://www.picotech.com/experiments/heat_transfer_coefficient/heat.html). Another gives a range of 5 to 35 for tubes in air at atmospheric pressure (http://www.cheresources.com/uexchangers.shtml). For comparison, the equivalent coefficient for radiation at about 20 C is about 6 W/m^2/K, found by taking the derivative of the Stefan-Boltzmann equation and evaluating at 300 K. By this comparison, convection dominates over radiation. Even if the convective coefficient is at the lower end of the range, convection and radiation are comparable. The link cited above from Hyperphysics (http://hyperphysics.phy-astr.gsu.edu/Hbase/thermo/coobod.html#c1) does not even attempt to estimate convection. The energy balance shown in the graphic is inconsistent, especially if convection turns out to be comparable to radiation.
The calculation in the article is far too conclusive. A more reasonable statement would be that radiation is significant, and probably comparable to convection. A full-blown calculation of the convective transfer rate would be required to say more.
Please forgive errors in form or style. This is my first comment here. Drphysics 19:32, 12 March 2007 (UTC)
Temperature of Earth
I did the computation in the formula several times and I kept getting 246.78 which is ~2 K lower than the 248.573 shown on the page. I may have done something wrong or I did not understand the formula properly. My calculation was done as follows: X=[sqrt(1-Albedo)]*Rsun Y=Sqrt(X/2D) Tearth = Tsun*Y
So? Who is correct? BTW a result in this computation should not have six signicant digits as the inputs are 3 or 4 digits.75.185.95.241 (talk) 16:34, 17 April 2009 (UTC)
- The numbers have been changed several times, e.g. here. It would be best to find source that does it out, and use their numbers, so that it's verifiable in a reliable source and we can see when it deviates from what the source says without rechecking the calculation. Dicklyon (talk) 21:44, 17 April 2009 (UTC)
- 75.185.95.241 your puzzlement about the Temperature of the Earth is not really surprising because the various formulas you can find for the same calculation generally include a term corresponding to the albedo. There are different opinions about what figure should be used for , this article has used various values over the years, currently it uses = 0.367 ; Detailed_explanation Greenhouse effect uses =0.3 and gets another temperature. There are links to be found here[[3]] with even more figures.
- Proper examination of the matter shows that Kirchoff's law of thermal radiation applies to planets, i.e. they are heated and cooled by radiation only, thus the temperature of a planet is independent of the albedo. This is true of all spherical bodies, a gold plated ball bearing would have the same temperature as the Earth, as would a glass marble. This truth is not accepted in greenhouse effect science which needs to sustain its thesis.--Damorbel (talk) 17:11, 18 April 2009 (UTC)
- That's incorrect, and a very bogus arguement by those who don't understand and want to deny greenhouse theory. The truth is that the absorptivity and reflectivity, though equal to each other, vary as a function of wavelength. Albedo is defined in the terms of the average reflectance and absorption of the incident solar spectrum, so it can be reduce to single number that determines how much energy the planet gets in from the sum. The emission, however, is more complicated, as the spectrum depends on temperature and the net emissivity averaged over the blackbody spectrum is therefore not constant. Greenhouse gasses are those gasses that are largely transparent to visible and near-IR solar radiation, but rather reflective (or absorptive and re-radiative) to the longer-wave IR of the planet's blackbody radition. This complexity is typical sidestepped by the definition of an effective temperature, which has a relation to actual temperature that is complicated by the greenhouse effect. What's being calculated here is an effective temperature, per its definition. Dicklyon (talk) 18:05, 18 April 2009 (UTC)
- @Damorbel Thank you for a thoughtful comment. Do you have a patio with direct sunlight and some white and black paint? I'm a novice at this thinking, but I wondered whether that would tell us anything. —Preceding unsigned comment added by 131.111.23.124 (talk) 05:53, 8 September 2009 (UTC)
- Incorrect? So a filter can generate or destroy energy? Before you go any further I suggest you read The Theory of Heat Radiation by Max Planck this nonsense that spectral effects, e.g. molecular resonance effects can create or destroy energy is becoming absurd. The (imaginary) blackbody effect supposedly posessed by the Earth means that energy disappears, arriving at one temperature and leaving at another without the possibility of disposing or aquiring energy in another way i.e. it is thermodynamically a closed system.
- When you have finished with Planck, you can read Einstein on the matter particularly "Emission and Absorption of Radiation in Quantum Theory," 17 July 1916 available here: [4].
- Can you not see that a body that absorbs only a fraction of the radiation incident on it is not going to be able to emit that absorbed energy with 100% efficiency? --Damorbel (talk) 21:13, 18 April 2009 (UTC)
- Yes, I can see that it won't emit at 100% efficiency. The point is simply that the relevant spectral band are nearly disjoint, so the emission efficiency is not very related to the albedo. That's one reason the "effective" temperature is used; it ignores emission efficiency and tells you only temperature of blackbody would emit that amount of energy. If you understand the definitions, your confusion should be cleared up. It doesn't require understanding at the level of Einstein or Planck, but is consistent with them. Dicklyon (talk) 21:30, 18 April 2009 (UTC)
- See details of wavelength-dependence and the greenhouse concept here. Dicklyon (talk) 21:39, 18 April 2009 (UTC)
- You can only get away with not reading Planck and Einstein if you work it out for yourself, you need a thorough appreciation of the mechanism of absorption and emission. All matters concerning heat are by definition about atomic/molecular motions. The spectral image in your link is only about the transmission of EM radiation, it does not indicate if the curves, very, very far from a "black body in the infrared" assumption! Your spectral graph is about transmission only, it takes no account of emission. But GHGs emit radiation just as easily as they absorb it; in a gravitational field they always emit more than they absorb for the simple reason that their density decreases with the distance away from the source (the direction of maximum emission).
- Molecules absorb/emit EM energy because they have an electric and/or magnetic dipole; there is no other EM mechanism. Because of the dipole moment EM waves interact with them by producing a mechanical force that accelerating them, the same force that makes an electric motor turn - no dipole, no absorption/emission! Gas molecules have mechanical resonances that give the absorption an irregular spectrum. A black body is a hypothetical concept comprising an array of resonating dipoles, it takes no account of the need for real matter, with its kinetic exchange of energy, to be present. Temperature is a purely kinetic concept, it is only the dipole moment that links it to radiation. --Damorbel (talk) 10:20, 19 April 2009 (UTC)
- Are there any sources that will help me understand your interpretation of greenhouse gasses; that is scientists who have worked it out and explained it? Are you saying that by invoking Einstein and Planck you can confidently prove that all planetary scientists are wrong? Interesting. I'd like to read more about that... Dicklyon (talk) 15:54, 19 April 2009 (UTC)
- The ones I gave are the classical ones but the whole matter was started by G. Kirchhoff in 1862 with his paper "On the Relation Between the Emissive and the Absorptive Powersf Bodies for Heat and Light"; available in English in the book "The Laws of Radiation and Absorption" by D B Brace, I found a PDF for free on line but have since bought a copy through Amazon.
- Kirchhoff's work was done 35 years before J J Thomson identified the electron as a particle, and even longer before Planck used it as a basis for his quantum theory and Einstein for his work on quantum mechanics. Like Newton's law of gravitation these works may be modified by new insights but never overturned completely.
- If I read you correctly, you agree that the ratio of emissivity to absorptance is unity throughout the spectrum then you are nearly there; the key to understanding is that absorbed incoming radiation heats the planet whatever its wavelength, it just so happens that the Sun is hotter than the Earth. The Earth radiates heat to deep space because it is hotter than deep space, it doesn't matter how it is heated. All that Kirchhoff noted was that it is exactly the same mechanism that causes the Earth to radiate as lets it absorb, the cooling and heating occur via the same means. Kirchhoff didn't know about electrons and molecular dipoles but he was right about the ratio of absorption and emission. I may be repeating myself but a planet with an albedo >0 can never behave like a black body, a requirement for the Greenhouse Effect
- I'm sorry that all those scientists who argue for the existance of a Greenhouse Effect do not seem to know about the radiation laws I am referring to but I can't help that, they never pursue the arguments fully but make various invalid assumptions, such as the Earth emitting as a black body, which give the wrong answer. --Damorbel (talk) 18:45, 19 April 2009 (UTC)
- I do understand all that you say about emissivity equals absorptivity at every wavelength. All the planetary scientists agree with that, too. But they don't misinterpret it as you do; the greenhouse effect is precisely because the planet cannot radiate as a blackbody; if it could, its temperature would be equal to the effective temperature, by definition thereof. Dicklyon (talk) 19:33, 19 April 2009 (UTC)
- Is it your argument that GHGs are what stops the planet radiating as a black body, i.e. they are responsible for the albedo? I am thinking that the reqirement for a black body is that it absorbs all incident radiation, i.e. it doesn't reflect any. --Damorbel (talk) 20:49, 19 April 2009 (UTC)
- Yes and no; , since they absorb in the band where a 255K or so emitter would emit; but no they don't affect the albedo much, since very little of the sun's energy is in that band. Under no condition should you think of the earth as a blackbody; there are many things that prevent it being one; it's still a useful comparative model though. Dicklyon (talk) 02:09, 20 April 2009 (UTC)
- Dicklyon - "GHGs reduce the earth's ability to radiate"? Not remotely true, H2O, CO2 etc. radiate powerfully in IR, just look here (Work your way to Meteosat 9 (full disk - WV), this shows just how much H2O (WV = water vapour) radiates. There is a wealth of images on this site.
- "Under no condition should you think of the earth as a blackbody" - that is what I have been banging on about. How can it be "a useful comparative model" when it predicts (for the Greenhouse effect) an equilibrium temperature of −18 °C ? This −18 °C (255K) is fully part and parcel of the CO2/H2O effect that is allegedly saving us from being frozen to death.
- It gets worse than that, the formula calculating this prediction is given in this article making the equilibrium temperature very strongly dependent on albedo, here it puts , giving a temperature of , very far from the 255K of the Greenhouse effect predictions. This alleged dependence of the planetary temperature on albedo can easily be shown to be nonsense, try putting albedo = 85% (like Venus), does anyone really believe that 2.5 times the cloud cover will drop the equilibrium temperature to 173K? --Damorbel (talk) 15:47, 21 April 2009 (UTC)
- You're very confused; the 255 K is not an equilibrium temp with greenhouse effect; it's WITHOUT any greenhouse effect; it's a good thing we have some greenhouse gasses or the planet would be very cold, as you seem to understand. For venus, the "effective temperature" considering the albedo would be very much lower than the actual temperature, since Venus has very strong greenhouse effect. Dicklyon (talk) 16:45, 21 April 2009 (UTC)
- I made no calculation including a greenhouse effect. I couldn't, I wouldn't know how to. All I say is the Earth's temperature is independent of the albedo because Kirchhoff's law of thermal radiation requires the emissivity/absorptivity ratio of planets to equal one . Didn't you agree with that? All I did was cite a formula used by the greenhouse faction, a formula that makes the Earth's temperature dependent on the albedo, a formula that does not have the ratio as one and therefore gives the wrong answer. --Damorbel (talk) 18:19, 21 April 2009 (UTC)
- I agreed only that aborptivity and emissivity are equal to each other at every wavelength; but weighted over the very different spectra of insolation and emission, they are essentially unrelated, as the plots in the books I referenced illustrate. Dicklyon (talk) 20:54, 21 April 2009 (UTC)
- The dominant fact remains that introducing the term (which has nothing to do with absorption and emission) into the temperature equation makes it thermally unbalanced and therefore invalid. Equally invalid is the concept that wavelength dependent effects can determine the totality of emission and absorption. The totality of thermal emission and absorption depends only on the exchange of EM energy and thermal (kinetic) energy by means of charge. Albedo arises from scattering/reflection processes which exclude energy exchange (mirrors are neither heated nor cooled by reflection). Reflection/scattering is redirection of energy without absorption, this distinction is never made in Greenhouse effect discussions, it is a shame that it renders the whole hypothesis invalid, it is the physics that is mistaken, not the mathematics. --Damorbel (talk) 08:36, 22 April 2009 (UTC)
- The factor introduced to account for wavelength-weighted absorptivity due to all those mechanisms is just ; you could call it anything different if you don't like to use albedo. In the effective temperature solution, it shows up as a fourth root (which someone decided to write as a square root of a square root. Since effective temperature is defined as ignoring emissivity, no corresponding factor for that is found to cancel it. If you want to know the temperature would be if average emmissivity equaled average aborptivity (each average over the corresponding relevant spectrum), then you can leave that out and you'll have an albedo-independent formula. But since we can measure and affect the albdeo and the greenhouse effect pretty much independently, that's not how it's usually done. Your "dominant fact" is nothing. Dicklyon (talk) 15:08, 22 April 2009 (UTC)
- The expression is how the promoters of the Greenhouse effect introduce the albedo into the calculation of the planetary temperature. The idea is that the reflected light, the light we see when looking at Earth from far away, is to be subtracted from the incoming solar radiation when calculating the temperature. Now if (the albedo), is 0.3 (30%) then the reflected part is the 30% and the absorbed part is (1-0.3)= 0.7 or 70%. Kirchhoff's law of thermal radiation states that the absorption coefficient equals the emission coefficient so there is (on average) a single temperature. If the Greenhouse hypothesis was correct and the Earth emmitted as a black body there would need to be be a real temperature and a second (lower) blackbody temperature (because a black body is a more effective radiator). Alternatively the planetary temperature would drop continuously because it was emitting more effectively than it was absorbing,--Damorbel (talk) 20:37, 22 April 2009 (UTC)
What makes you think the Earth as a whole is in thermal equilibrium? The amount of solar radiation incident on the Earth changes radically between winter and summer, so it is a driven oscillator even on short time scales. And there was increasing amount of solar energy trapped in stored oil over hundreds of millions of years, which we are now releasing in a (geologically) short time, and lots of other things driving the system in a nonequilibrium fashion. For that matter, all of biological evolution represents a nonequilibrium situation. Of course, we will eventually reach equilibrium at the heat death of the universe, but until then arguments about the planetary climate based entirely on thermal equilibrium seem bogus. In practice, we only ever have approximate thermal equilibrium locally, on smale scales of space and time (compared to the scale of the planet and geological time). And claiming that thousands of climate scientists have never made detailed studies of the radiative forcings in the system is absurd (and contradicted by mountains of publications...see the IPCC report for a summary). —Steven G. Johnson (talk) 16:54, 22 April 2009 (UTC)
- Steven G. Johnson I am perhaps mistaken but I suppose it is me you are referring to. Let me say straight away, you are entirely correct in pointing out heat sources and sinks that cause deviation from thermal equilibrium. Perhaps you should take account of day/night variations also. The biological and fossil fuel effects you mention can best be calculated separately and, in so far as the will cause deviations in the average temperature, one must know the actual emissivity (absorptivity still equals emissivity) of the planet to find out the rate of cooling/heating. This is in stark contrast to the unity ratio that applies when calculating the temperature due to the Sun's radiation, as I said before, the unity ratio comes from the fact that radiative emission/absorption is by means of the same atomic/molecular charge, there is not one set of molecules/atoms emitting and another set absorbing.
- I know I refer to "thermal equilibrium" which, strictly speaking, means there is no heat flow but in fact there is a great deal of heat flowing from the Sun. Steady state is a more accurate description. --Damorbel (talk) 17:28, 22 April 2009 (UTC)
- "there is not one set of molecules/atoms emitting and another set absorbing" you say. But to a large extent, that's false. The involvement of differnet molecoles in the atmosphere and solids and liquids depends on their particular transitions; the transitions that do most of the absorbing of solar radiation are those matching the visible and near-IR and near-UV wavelengths where most of the solar energy is. The molecules involved in radiation are those that better match the approximate black-body spectrum of the planet. To a large extent, they are not the same molecules, though of course there's a lot of overlap, especially in the solids and liquids where the transitions are very spread out. Dicklyon (talk) 18:00, 22 April 2009 (UTC)
- "The involvement of differnet .... where most of the solar energy is." Again yes! But the concept of a single temperature for the whole planet is also quite wrong but not misleading. The concept that the albedo influences the average planetary temperature is not only incorrect but misleading, it is dangerously wrong since it is causing money to be wasted on costly projects to save us from something that isn't going to happen, namely planetary temperature change due to CO2 in the atmosphere!--Damorbel (talk) 18:20, 22 April 2009 (UTC)
- How can you assume that the Earth is in a steady state? This is not true on either short time scales (day/night, winter/summer) or on longer time scales (there is clear evidence of many kinds of long-term temperature variations). —Steven G. Johnson (talk) 22:15, 22 April 2009 (UTC)
- The "assumption" is not expected to represent anything real; it's a calculational device, like "effective temperature" is; the thing that's easy to solve for is what would be the one temperature, which if constant, and if the earth radiated like a blackbody, would lead to radiation that matches the energy absorbed from the sun. It's not mysterious, but also not intended to be realistic. It's just what it is. Dicklyon (talk) 00:02, 23 April 2009 (UTC)
- Since we agree that this assumption is unrealistic, and hence the results it produces are misleading, why is there a huge section of the article on black bodies devoted to this misuse of equilibrium assumptions? —Steven G. Johnson (talk) 15:35, 23 April 2009 (UTC)
- The results aren't misleading if sensibly interpreted; we have this section because it is a common approach in reliable sources. The "effective temperature" gives you an idea what the temperature would be if the planet radiated as a blackbody. The difference relative to the real average temperature is mostly due to greenhouse gasses in the atmosphere. The method is one step toward understanding that. Dicklyon (talk) 16:04, 23 April 2009 (UTC)
- Steven, "steady state" is not an assumption, it's an observation. Historically the solar system has a beginning, a life and an end, effectively its state is changing all the time. In any given period, much shorter than the total life, there is an average or "steady" state, during this period there will almost certainly be deviations from this "steady" state. But deviations observed, large or small, will probably have an identifiable cause, thus the benefit of identifying a steady state comes from discovering a cause of deviations, be it a little CO2 in the atmosphere or a supernova event in the Sun. --Damorbel (talk) 07:44, 23 April 2009 (UTC)
- In other words, it's not in a steady state, because there are numerous causes of unsteady behavior. Hence arguments against greenhouse effects based on "equilibrium" considerations are bogus (and, for that matter, circular: if you assume it's in steady state, then by assumption there can be no heating). —Steven G. Johnson (talk) 15:35, 23 April 2009 (UTC)
- The notion of an equilibrium or steady-state solution is useful, and not bogus, even in the context of systems that are not quite in equilibrium and not quite in steady state. But you're right that many of the arguments against these analysis are bogus. Those who want to argue that greenhouse gasses are not the reason why the planet's average temperature is higher than the calculated equilibrium effective temperature need to show other reasons why the net emissivity is as low as it is; Damorble argues that it's lower because it should be equal to 1 minus the albedo, just like the absorptivity is; but his argument is bogus because it appeals to a misinterpretation of the fundamental physics by ignoring the wavelength dependence; it also ignores all that's known about planetary atmospheres; he pretends that his amateur analysis is better than the long-evolved understanding of the planetary science community. This is a typical argument by people whose real objection is political or religious, rather than scientific. Please note that I'm not among those who think that the scientific point of view ("SPOV") needs to trump other points of view in wikipedia; but so far I haven't seen any other points of view of this question in reliable sources; if there's a religious or political organization that has published an analysis the differs, in a reliable source, I'd have no issue with mentioning and citing that alternative POV. Dicklyon (talk) 17:35, 23 April 2009 (UTC)
- Dicklyon, you never made it clear what is your source for Kirchhoff's laws, since he explicitly excludes wavelength sensitivity your argument that it has a wavelength sensitivity does not carry a lot of weight. What papers have you read on the matter, I would like a reference. I have read many of the publications of what you call "the planetary science community" and following an old tradition, I am pointing out an inconsistency. You make remarks that I am an amateur and use "bogus arguments", I think you intend these to be deprecating attacks on the quality of my contributions. If this is so, may I ask you to withdraw them? Steven may consider withdrawing his similar remarks. Thanking you in advance. --Damorbel (talk) 06:09, 24 April 2009 (UTC)
- No, his argument is bogus because he's mis-applying Kirchhoff's law to a system that is not at thermal equilibrium, not even locally on the relevant lengthscale of the ground/atmosphere system. —G. Johnson (talk) 21:44, 23 April 2009 (UTC)
- Steven, what is your source for Kirchhoff's law of thermal radiation? This is a very nice article but it has its limitations. Have you read the original papers? It was Kirchhoff who introduced the concept of a black body, you should have read what he has to say on the matter before describing anyting as "bogus". Have you read Max Planck on the matter, or Albert Einstein. These sources are a suitable introduction. If you have read them you will be able to say precisely where I misunderstand them. Until you have read them, I suggest you will have difficulty in explaining why the equation quoted from this article, is mistaken and that the term is the cause of the error. --Damorbel (talk) 06:09, 24 April 2009 (UTC)
- Damorbel, you are correct in observing that my remarks represent "deprecating attacks on the quality of [your] contributions". That's because your "contributions" are stupid and pig-headed. I'm sure you're a great guy, fun to have a beer with and all, but what you keep saying is nonsense; your pretense that your analysis is "pointing out an inconsistency" in the planetary science is pure hogwash. I hope you don't misinterpret me and think I would take any of this back. Dicklyon (talk) 06:32, 24 April 2009 (UTC)
- Stupid? Pig headed? That is how you see my objection to the equation ? I have given good sources to show it is invalid, you don't appear to have read them and you respond with abuse! Is this your idea of good wiki practice? --Damorbel (talk) 07:59, 24 April 2009 (UTC)
- No, I admit that I continued to respond long after it was useful. Dicklyon (talk) 15:20, 24 April 2009 (UTC)
This article is a prime example of why Wikipedia cannot be trusted as a definitive source for , particularly , politically sensitive physics . The modification of the correct black body equation by adding an albedo term to the earth's absorptivity while blatantly failing to add the corresponding term for its emissivity is inexcusable . I'm glad to see Damorbel asserting exactly the same basic classical physical arguments I have made both here and on the Stefan-Boltzmann page over the last couple of years . That Wikipedia allows the absurd assertions that the albedo of a uniformly colored sphere will effect its equilibrium temperature , or that that doesn't matter because the earth is always just chasing its equilibrium , is a black mark against Wikipedia . I have implemented the correct classical physics at http://cosy.com/Science/TemperatureOfGrayBalls.htm . This simple implementation provides quantitative , experimentally falsifiable albeit sensible values . As pointed out above , leaving out the emissivity term for the earth leads to absurd results . Wikipedia needs to get a real physicist to clean up these pages despite however much they pray for the "greenhouse effect" to be real . Right now , this page is an embarrassment . Bob Armstrong (talk) 22:15, 25 April 2009 (UTC)
- The person who should be embarrassed is you. Physicists are well aware that emissivity or absorptivity will not affect the equilibrium temperature if it's the same at all wavelengths; they're also well aware that's it varies strongly with wavelength and that albedo is just a weighted version, reduced to one number based on the solar spectrum, and that it's pretty much unrelated to a similarly weighted version using the blackbody emission spectrum. They define an "effective temperature" not to be absurd, not to calculate a meaningless number, but to calculate a baseline against which to compare the actual temperature that depends on the emissivity. They all know the earth is warmer than the "effective temperature" because it does not radiate like a blackbody. They've also studied the reasons for that in great detail, and have found the gasses in the atmosphere that absorb those radiated wavelenghts and re-radiate much of it back to the earth are part of the reason. They call these greenhouse gasses. Nothing here is political or religious until you get into people like you who are confused at the difference between greenhouse gasses and anthropomorphic greenhouse gasses, and have political or religious reasons to want to deny the latter and get confused and also deny the former. Yes, I'd be very embarrassed. Dicklyon (talk) 22:25, 25 April 2009 (UTC)
- Your linked page includes the following bizarre statement: "The 'Black Body' Wikipedia page was the first place I saw the derivation of the Earth's temperature from the Sun's based on this equation." Bizarre, why? Well, glad you asked. Two things: first of all the wikipedia page does NOT calculate the Earth's temperature using this equation; it calculates only the "effective temperature", which is very clearly stated not to be the actual temperature; it references what the term means, very clearly. Second, it is bizarre that you would write a page like this, pretending to be some kind of a physics or planetary science expert, never having read any of the books about this kind of calculation. I first saw this in college over 35 years ago; greenhouse effect and understanding of planetary atmospheres and such long predates the concern over anthroporphic global warming that you seem to be so allergic to. Dicklyon (talk) 22:32, 25 April 2009 (UTC)
- Just noticed your funny footnote that includes "I have seen it pointed out that Kirchhoff's equating of absorption and emission only applies at equilibrium. However absorption and emission spectra are generally rather constant for a substance over a given physical state." This gives me a clue what Damorbel was going on about and what some of the discussion above about equilibrium was being driven by. It's true that the spctrally averaged emissivity and absorptivity would be equal in an equilibrium situation, since the radiation being emitted and absorbed would be of the same black-body temperature. This is nowhere near true in the case of the radiation from the sun impinging on the earth. As several sources that I linked above show, the situation with the sun around 6000K and earth around 300K is so far from equilibrium that the spectra are almost totally disjoint. All your examples about grey spheres will have the same problem; the actually steady-state (not equilibrium) temperature of your balls in sunlight will depend not at all on how dark or light they look, but rather on how that albedo compares to their long-wave IR emissity. Maybe you need to do that experiment instead of the gedanken one you describe as "If it were the case, one would expect a ball coated with Magnesium Oxide with an albedo of about 0.9 to come to an equilibrium temperature of about -120c in a vacuum bottle sitting in room temperature surroundings," which simply displays your total failure to comprehend. Dicklyon (talk) 22:44, 25 April 2009 (UTC)
- With respect to Venus you also have the absurd statement "The most heating this greenhouse theory could predict is raising the temperature back to the black body temperature. No 'runaway' effect could raise the temperature beyond that." What you mean here by "the black body temperature" is not even clear; I suppuse you mean the effective temperature that would have been computed when ignoring albedo? In any case, it's nonsense; the actual temperature can be any amount above the effective temperature; there's nothing that constrains the net emissivity to be greater than net absorptivity. Venus is in fact a good example of where greenhouse theory works out well to explain what would otherwise be uninterpretable, or interpretable only as an unknown internal heat source as you say "Confirming that Venus is much hotter than any simply radiantly heated object in its orbit could be . There must be some other internal source of heat." Your "confirmation" would be better called a "hallucination" or "divine inspiration" or "bullshit" or something like that. Your admission that "I do not understand how James Hansen could possibly have claimed that Venus's mean temperature could be due to heat trapping of any sort" begins to make sense... Dicklyon (talk) 23:00, 25 April 2009 (UTC)
No , you've now had multiple people point out the absurdity of your neglect of Kirchhoff . Your second sentence in your reply is simply wrong . albedo has nothing to do with solar or any other particular spectrum . It is simply the assumption of a flat spectrum . All the stuff about "effective temperature" is irrelevant obfuscation . It refers to the apparent temperature of radiant bodies observed from the outside . If you agree that the earth does not radiate as a black body , how can you possibly justify leaving the parameter out of the equation ? Forget any gasses or detailed structure . This is about the radiative equilibrium of balls depending on their temperatures and their spectra . It doesn't matter how the spectra are produced , all that matters is what the spectra of the bodies are . Until this section of the page got perverted by the unjustifiable addition of an absorptivity parameter to the left side of the equation while leaving the emissivity of the right side as a black body , the equation was correct . With your distortion , as has now been pointed out by others also , a white ball will be colder than a black ball in exactly the same radiant environment . Show me any experimental verification of that effect . You can't because it's absurd . It doesn't matter what either of us have read or not read , tho I am quite happy for anyone to judge me by my http://CoSy.com . What matters is LOGIC and experiment . Energy is conservative . Therefore the average over a cycle is equivalent to an equilibrium .
I know precisely how to extend my implementation of the Stefan-Boltzmann/Kirchhoff to spectra and expect to do it as I find time this year coming out with quantitative , experimentally testable numbers . So far as I can tell , you don't have a clue how to extend your analysis to spectra ( and your "greenhouse" gasses ) . In any case , the quantitative extension of your analysis to spectra is nowhere to be found on Wikipedia . Your statement that "with the sun around 6000K and earth around 300K is so far from equilibrium" shows such a lack of comprehension that it should by itself disqualify you from having control of any of these pages . It's your equation that predicts the absurdity of white balls being cold because they are white , not the correct equation .
The meaning of "black body temperature" in my paper is what is calculated by the Stefan-Boltzmann/Kirchhoff equations I implement . I'm talking about real temperatures that can be measured by real thermometers or determined by measuring radiant flux . The fact that you do not even understand that Venus is radiating on the order of 16 times the amount of energy that an object in its orbit is receiving from the sun shows your total incompetence . Actually in Hansen's defense ( only on this issue , not his general behavior ) I recently saw some comment that he did talk about some internal heat source in Venus in his original work .
I will repeat , this page is an embarrassment to Wikipedia . I again challenge you to present any experimental verification that albedo of a radiantly heated ball affects its mean temperature . I further challenge you to present the next steps in calculating the effects on temperature of differences in spectra , as with various gasses , of radiantly heated balls . My implementation has already been replicated by another person and has been translated into a more accessible array language which anyone can download and experiment with or extend .
This nonsense has been allowed to fester in Wikipedia long enough . I appeal to the masters of Wikipedia to enlist some real thermodynamicists to clean up these pages with experimentally verified and rational physics . Politics and religion should have nothing to do with it . Bob Armstrong (talk) 00:00, 27 April 2009 (UTC)
- Albedo can be defined either as a wavelenght-dependent thing or as a weighted average, as in "broadband albedo is the reflectance in multiple bands integrated over the total solar spectrum." This depends on the solar spectrum. What definition do you prefer? Or do you ignore the definition, as you do with "effective temperature"? Dicklyon (talk) 01:57, 27 April 2009 (UTC)
- Dicklyon, Albedo is a reflection/scattering process; as such it can introduce delays in the lightwave propagation producing spectral (colour) effects by interference, as well as by selective refraction. You should also realise that an important component of albedo comes from the incident wave encountering a change in density, another factor mostly independent of temperature and certainly having nothing to do with absorption and emission. Closely related is diffraction (see Diffraction grating). These (spectral) effects are quite independent of temperature. The magnitude of thermal absorption and emission depend only on the interaction of an EM and charge or a dipole, I have probably pointed out before that this occurs at a dimension far below optical wavelengths so it is not wavelength sensitive. The wavelength properties of the body confuguration govern the spectra of emission and absorption but they cannot alter the total energy.
- The question of effective temperature is relevant. Effective temperature is the temperature of a body deduced from its radiation "signature", i.e. by measuring the output of EM energy. Take two cases, a gold plated sphere which reflects 99% of incident radiation i.e. it has an albedo of 0.99 and its absorbtivity is 0.01; take a charcoal sphere that reflects 1% (albedo 0.01) and absorbs 99% of incident radiation, put them in the same orbit as Earth and let them rotate so that they get to an even temperature. Now, if the nominal temperature is 283K, what is the effective temperature of the gold plated sphere and what is its thermal temperature? I think you will find that, being such a poor emitter, the gold sphere will be almost invisible in the infrared but is thermal temperature will be just the same as the charcoal. The charcoal will of course be very much brighter in the infrared, whereas the gold, with its higher albedo will be much brighter, if you are able to see it (c.f. Venus).--Damorbel (talk) 15:03, 27 April 2009 (UTC)
- As to the balls you have, the equations correctly predict that the white one will have a much lower "effective temperature" than the black one, since the white one will absorb less energy from the sun, and therefore in steady state will emit less energy, and since the "effective temperature" is simply a measure of that emitted energy (the temperature of a black body that would emit the same amount of energy as a white sphere is going to be much lower than the temperature of the white sphere itself); if you don't accept that definition, and choose to keep erroneously confusing "effective temperature" with temperature, then of course you're going to have this conflict that you seems to torment you. Dicklyon (talk) 02:02, 27 April 2009 (UTC)
- Your challenge to "present any experimental verification that albedo of a radiantly heated ball affects its mean temperature" is irrelevant, since the equations here don't say anything about mean temperature, only about effective temperature. Yet different colors and textures of ball do have different steady-state temperatures in the sun, depending on how the average absorptivity in the solar spectrum (1 – albedo) compares to the average emissivity in the band relevant to the actual radiation at a much lower temperature than the sun. That's why on your page showing thermometers in balls in the sun, the shiny one has a rather different temperature than the others; whether the black on or the white one comes out warmer depends on the emissivity of those surfaces at longer wavelengths than you can see. Dicklyon (talk) 02:11, 27 April 2009 (UTC)
- Dicklyon, the Greenhouse effect, because of its thesis of "blackbody in the infrared", claims that, for the Earth, without the radiation effects of H2O,CO2 etc. would have a temperature of 254K. To justify this they use the albedo in their calculations, but the albedo is not a radiation effect of CO2 H2O etc., it is a reflection/scattering effect of the entire planet, an effect not related to thermal absorption/emission.--Damorbel (talk) 15:31, 27 April 2009 (UTC)
- Don't worry, I don't have control of any of these pages. If you'd like to help, I suggest you try to understand reliable sources first; here is a good book that's very clear on the definitions and why the average emissivity and absorptivity are different so that it is "possible and convenient to treat the solar and terrestrial fluxes independently." And here's a book that should help clear up your delusions about Venus. Dicklyon (talk) 02:11, 27 April 2009 (UTC)
Your book contains the mistake I am talking about, I have seen many better argued but still incorrect publications, their better argument doesn't make them correct. I can find you books that say the Sun rotates round the Earth, that heat is phlogiston, even Sadi Carnot believed it was a substance called "caloric". For years "the vast majority of scientists" believed light travelled through a substance called Aether, they wrote books about it; the greenhouse effect is much the same, the "theology" of global warming says CO2 must be the cause,even if thermodynamics says it cannot be!--Damorbel (talk) 15:31, 27 April 2009 (UTC)
- I've followed this whole discussion, and I pretty much have one point to make. If the greenhouse effect is a load of crap, why, pray tell, are there greenhouses? Dicklyon's physics apply equally well to both greenhouses and earth. The other two guys' versions are, from what I can tell, things made by people who've read something but didn't quite understand it (but who think they did). Headbomb {ταλκκοντριβς – WP Physics} 06:32, 27 April 2009 (UTC)
The temperature of the Earth with no atmosphere.
I wrote a small comment on this at 11.25 AM on 7 sep 09 and placed it higher up on this page. I was trying to be polite. But I have now scrolled down and have had a glimpse of a nightmare i.e of what Wikipedia might have become.
As I implied earlier you can make any approximation you like in a model, but not all models are equal. Some of them are totally unrealistic. The emissivity (1-alpha) of solids in the infra-red is usually about 1 so it is sensible to ignore the albedo in that frequency range. In the visible range you can set (1-alpha) =1 but if you do , you would have to predict that photographs of the Earth from outer space would not look mainly bright blue but would be completely undetectable unless you had an infra-red camera.
It is rather like using a steam hammer to crack a nut, but if you are unsure a good reference for Kirchoff's law is Landau and Lifshitz, Statistical Physics ,1958, p.176. As stated by others earlier on, you cannot combine the Sun and Earth into a single equilibrium system without raising the Earth to about 6,000 degs.C. The same applies when you add an atmosphere which is not at the same temperature as the ground. In effect the emissivity for a layer of CO2 or H2O is highly variable across the IR range. That does not mean that Kirchoff's law has broken down. —Preceding unsigned comment added by Deconvoluter (talk • contribs)
I'm tired of this greenhouse effect nonsense. What temperature would the atmosphere be if it were transparent (contained no CO2 and no water)? It would be as warm as the earth, because transparent gas does not radiate. The only reason it's not warm is because CO2 and water allow it to radiate its heat out into space. This nonsense about the absorption of CO2 sending heat back to the earth, or whatever, violates the second law of thermodynamics, because it assumes that the atmosphere without CO2 and water would be cold. But no gas can be transparent AND fail to radiate.Kevan Hashemi 23:05, 7 January 2010 (UTC) —Preceding unsigned comment added by Kevanhashemi (talk • contribs)
Hi all. I appear to have stumbled into a months-old conversation, but I want to chime in because the topic is so important, and the misconceptions apparently so deep. Before I make my argument, here's a case to ponder: Why is the surface temperature of Venus higher than the surface temperature of Mercury? Mercury is much closer to the sun.
Dickylon's argument is correct, but it seems that he has not been able to communicate it effectivley enough. Let's consider the Earth-sun system. The sun radiates at all frequencies, but dominantly in the 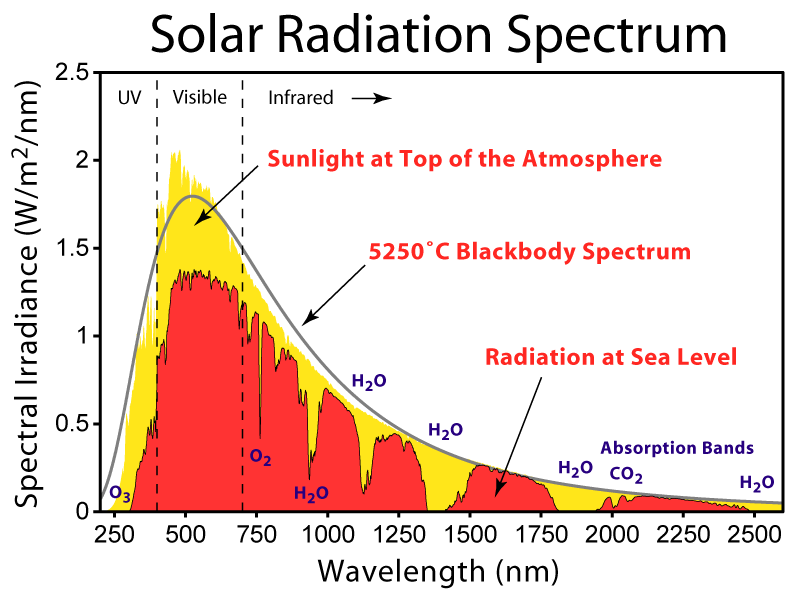 range. The atmosphere is primarily transparent to visible light, although the visible light is slightly attenuated as it passes through. Most of the visible light will reach the Earth's surface, and much of this will be absorbed - although a significant amount will be reflected, due to the Earth's albedo. As Deconvoluter says, this reflected light is the reason why the Earth appears bright blue from space.
range. The atmosphere is primarily transparent to visible light, although the visible light is slightly attenuated as it passes through. Most of the visible light will reach the Earth's surface, and much of this will be absorbed - although a significant amount will be reflected, due to the Earth's albedo. As Deconvoluter says, this reflected light is the reason why the Earth appears bright blue from space.
If the system were to be in thermal equilibrium, by the proper definition, then the Earth would be at the same temperature as the Sun, and radiate light mainly in the visible spectrum as well. As the emissitivity equals the absorptivity for any frequency, the transparent atmosphere would radiate a small proportion of this light, and most would come from the surface. That light would pass through the atmosphere, and the Earth would behave effectively as a gray body, in accordance with the claims of users Damorbel and Bob Armstrong. Its temperature would not depend on the albedo in this equilibrium circumstance, a clear result of Kirchhoff's_law_of_thermal_radiationKirchoff's law.
However, the Earth and Sun are evidently not in thermal equilibrium (which is by no means the same as steady state, as Damorbel pointed out). In fact the Sun transfers a great deal of heat to the Earth, and the Earth transfers very little heat back to the Sun. The surface of the Earth is not as hot as the surface of the Sun. As a result, the system behaviour cannot be accurately treated as a grey-body problem.
Incident solar radiation is primarily in the visible range, and the temperature of the Earth's surface is roughly perhaps 300 Kelvin. At 300 Kelvin, thermal radiation is mostly emitted in the infrared range and below. So we should all agree that the incident solar radiation spectrum, and the emitted terrestrial thermal radiation spectrum, have completely different wavelength compositions. The overlap between the two spectra is small. However, as the system is in an approximately steady state (as opposed to equilibrium), the total power of both absorbed incident and emitted outgoing radiation is the same. Hence, the absorbed incident spectrum (primarily visible), and the outgoing spectrum (primarily infrared), have the same power.
(Are we all still in agreement? I hope so. I haven't said anything controversial yet).
As was duly pointed out by Damorbel, the atmospheric gasses emit powerfully in the infrared spectrum. And, we all agree that the emissitivity of these gasses is the same as their absorptivity, so they will also absorb powerfully in the infrared range. Hence, the atmosphere will absorb terrestrial radiation from the Earth. This should not be controversial. For any given small volume of atmosphere of homogeneous temperature, radiative emission occurs in equally all directions. The atmosphere will therefore emit radiation both into space and also back to the surface of the Earth. Radiation will be emitted and re-absorbed by the atmosphere several times, with a net flow of radiation outward into space. Because of the opaqueness of the atmosphere, the thermal radiation emitted by planet Earth will primarily have been emitted by the atmosphere. As well, because of the opacity of the atmosphere at infrared wavelengths, much of this will have come from the upper layers of the atmosphere. The total power being radiated into space is still equal to the absorbed incident power on the Earth's surface. Are we all still in agreement?
There is a temperature gradient in the atmosphere. The atmosphere is typically warm at the surface of the Earth, and cold at high altitudes. This too should not be controversial. In fact this temperature gradient is by and large a consequence of the discussion above, where solar radiation is absorbed by the Earth's surface but terrestrial radiation is emitted from the atmosphere. However, regardless of the cause of the temperature gradient, it is clear that the upper atmosphere is cooler than the surface of the Earth. We also have concluded that terrestrial radiation is emitted from the atmosphere.
Albedo aside, the existence of the greenhouse effect should be very apparent from this discussion. A power balance has shown that the incoming absorbed solar radiation equals outgoing atmospheric radiation, in the steady state. This power balance can yield the temperature of the upper atmosphere. The surface of the Earth is warmer than the upper atmosphere. This additional warmth is called the "greenhouse effect", and is a consequence of the fact that the atmosphere can be transparent to visible light (which strikes the land and sea), but opaque to infrared light (which is emitted by the land and sea). This effect can only be observed when the system is not in equilibrium, as I explained above.
Thank you for reading patiently. --JB Gnome (talk) 09:26, 22 November 2010 (UTC)
Would it be possible to change this derivations symbols so as to avoid all these horrible subscripts? Larryisgood (talk) 22:12, 2 April 2010 (UTC)
A "cloud of light"??
What is a cloud of light? Is this terminology really used by scientists? Wouldn't it make more sense to say it's surrounded by a box at the same temperature? —Preceding unsigned comment added by 71.167.71.186 (talk) 01:48, 7 December 2009 (UTC)
- It is a manifestion of the weird science in this article. [5] is equally weird, taken to its logical conclusion this explanation is good for perpetual motion. But the item is robustly defended because it is an important factor in the Greenhouse Effect, so it must be true. --Damorbel (talk) 12:25, 7 December 2009 (UTC)
- I've never heard of it. It seems more likely to be a typo for "cloud of gas". I'll change; change back if you object and can explain. My undergrad heat transfer course and grad combustion course never mentioned "cloud of light". —Ben FrantzDale (talk) 13:28, 7 December 2009 (UTC)
- Yes, but a cloud of gas would need some source of energy to make it glow, examples are discharge lamps and the Earth itself emitting IR.--Damorbel (talk) 16:47, 7 December 2009 (UTC)
- It's an unusual phrasing, but it's not a typo for cloud of gas (in the sense of a gas made up of atoms or molecules). In a more technical context it might be called a Photon gas, but I can see why someone might want to use a more descriptive term in an introductory article. Djr32 (talk) 19:50, 7 December 2009 (UTC)
A Photon gas has really got very little connection with a "real" gas, particularly when thinking in astrophysical terms. Photons have no mass so they have no tendency to form clumps through gravitational attraction, they do not collide with each other at all therefore they cannot exchange energy like a "real" gas. Most observations about photon gases put them in a cavity, either reflecting or "black". If the cavity reflects 100% (!) they do not change their energy with time; if it is black photons have a very short lifetime, about the transit time accross the cavity; they are emitted and absorbed by the walls and thus their energy distribution quickly adopts the spectral characteristic of the cavity and its walls. --Damorbel (talk) 20:47, 8 December 2009 (UTC)
- As I mentioned in response to an earlier poster, "thermal bath of photons" might be a more standard terminology in this context. — Steven G. Johnson (talk) 02:31, 9 December 2009 (UTC)
- It would be more logical to say "a bath of thermal photons". --Damorbel (talk) 19:30, 30 December 2009 (UTC)
- A quick google search reveals that "thermal bath" is far more common. — Steven G. Johnson (talk) 20:18, 30 December 2009 (UTC)
Contradiction
I have made a small revision in this section to draw attention to the contradiction between the stated properties of a Black body and the calculation of planetary temperature. --Damorbel (talk) 07:08, 31 December 2009 (UTC)
- I wasn't logged in when I reverted it (since I'm supposed to be on wikibreak), but that was me. I think this seems like a continuation of your campaign above to deny and/or obfuscate the idea of a greenhouse effect, by refusing to accept the definition of effective temperature. The assumptions are stated, and do not include the idea of a blackbody reflecting radiant energy; the planet reflects radiant energy, but it's clearly not a blackbody and not treated as such; the effective temperature is the temperature that a blackbody would have, to emit the same total power as the planet; nothing more. I updated "blackbody temperature" to "effective temperature" in the box to make it more clear and correct; there is no contradiction. Dicklyon (talk) 21:24, 31 December 2009 (UTC)
Vague
Vague statements like this have no place in a Wiki article. --Damorbel (talk) 09:59, 7 January 2010 (UTC)
- That type of statement exists because people confuse the definition of the Greenhouse Effect with the theory that explains how the Greenhouse Effect works. Basically, the dark parts of the Earth are much warmer than the dark parts of the Moon. The Greenhouse Effect is simply the phrase used to describe this difference in minimum temperatures. Since the most obvious difference between the bodies is the atmosphere of the Earth, it is logical to assume that somehow the Earth's atmosphere is responsible for the temperature difference. However, most people are not aware that there are several different theories on how the atmosphere keeps the surface warm.
- There is also the problem of average temperature. Is the average increasing because the minimum temperature is increasing, because the maximum temperature is increasing, or both? This makes a big difference in predicting if the current increase will melt ice and produce havoc. However, this is not the correct article for this level of detail, and the other articles won't allow even the hint that the science could be other than presented by the IPCC. Q Science (talk) 19:29, 7 January 2010 (UTC)
"Basically, the dark parts of the Earth are much warmer than the dark parts of the Moon." Not really true for many reasons, 1/ the lunar period is about 29.5 earthdays so the lunar night is nearly 360 hrs long giving it a long time to cool. 2/ on Earth the atmosphere spreads heat partly through the thermal gradient between the equator and the poles and partly because of the Earths relatively rapid rotation. The seas do a similar thing with probably greater effect since their mass is somwhere near 600 times that of the atmosphere. Because of their properties as fluids, the oceans and the atmosphere, besides spreading the heat, also have a thermal inertia effect that prevents rapid temperature change and severely limits temperature excursions when compared the extreme Sun/shadow (250C?) excursions found on the moon. The so called Green house effect is not involved in this at all. --Damorbel (talk) 21:19, 7 January 2010 (UTC)
- 1) The moon is in the dark for 29 days, the Earth's poles are in the dark 6 months (over 150 days). The Greenhouse Effect is what keeps the poles about 200C warmer than the Moon. Q Science (talk) 21:38, 7 January 2010 (UTC)
- 2) What you describe in point 2 IS the Greenhouse Effect. By definition. Why do you claim otherwise? Q Science (talk) 21:38, 7 January 2010 (UTC)
- "The Greenhouse Effect is what keeps the poles about 200C warmer than the Moon." Sorry, but no it isn't. It is the circulation of air from warm(er) regions via the Hadley cells that warms both poles. In addition for the North pole the circulation of warm sea water makes a major contribution. The ocean in the North polar region is up to 4000m deep. To see how powerful this is look at Greenland. Greenland has no water underneath it, just like the South pole, that is the main reason it is covered with ice, in addition much of Greenland is more than 2500m asl, keeping it about 16C below the surrounding sea.
- "What you describe in point 2 IS the Greenhouse Effect" I'm afraid not. The effects I describe do not need any trace gases such as CO2, H2O, CH4 etc., the sea and the atmosphere are heated by common thermal processes such as insolation, convection and evaporation/condensation.--Damorbel (talk) 10:07, 8 January 2010 (UTC)
- "IS the Greenhouse Effect. By definition." Really? I thought the GHE was something to do with Greenhouse gases.--Damorbel (talk) 10:11, 8 January 2010 (UTC)
- Yes those are all a part of the Greenhouse Effect. However, the key is that the only way for that heat to get back to the surface is when it is released by Greenhouse gases. CO2 and water vapor return heat to the surface. Without those, the atmosphere would be at a constant high temperature and the surface would be much colder.
- "only way for that heat to get back to the surface" What does this mean? Surely most of the Earth's heat arrives at the surface by direct sunlight?--Damorbel (talk) 12:12, 9 January 2010 (UTC)
- By definition, the Greenhouse Effect is everything that makes the surface temperature of the Earth different than the surface temperature of the Moon. Q Science (talk) 20:38, 8 January 2010 (UTC)
- So CO2, H2O etc. are not really necessary for the greenhouse effect?--Damorbel (talk) 12:12, 9 January 2010 (UTC)
- Some heat comes directly from the Sun. Some heat is stored in the atmosphere and released back to the surface by CO2 and water vapor. Without Greenhouse gases, heat would still be added to the atmosphere by convection, but there would not be any way to get rid of it. Convection can not release heat to space. The only way for heat to get to space is via IR radiation. The only way for the atmosphere to transfer heat to the surface is by IR radiation. Besides Greenhouse gases, dust is also able to radiate heat. Q Science (talk) 20:22, 9 January 2010 (UTC)
Temperature relation between a planet and its star - This is wrong
Basically this derivation says that by conservation of energy, incident = reflected + emitted.
where is the radiation falling upon the earth from a black-body sun at temperature , is albedo and is the black body radiation of the earth at temperature . This equation violates Kirchhoff's law of thermal radiation. A black body cannot be reflective. A better analysis would be to assume that the Earth is a grey body with absorptivity and emissivity , which are equal by Kirchoffs Law. Albedo is simply part of the reflectivity which is .
By conservation of energy:
or
Solving for the Earth's temperature yields
which, using the given parameters gives an Earth temperature of 5.517 degrees C. Greenhouse effects will, of course, raise this temperature. Note that this analysis cannot be used for the moon, because of the wide swings in temperature on the light and the dark side. Since black body radiation is not linear in temperature, but rather the fourth power of temperature, this means that the moon will be radiating (losing) much more energy on its light side than its dark side, causing the effective temperature to be lower.
I don't want to replace the present referenced (but incorrect) analysis with an unreferenced one without consensus. PAR (talk) 21:15, 9 January 2010 (UTC)
- The uv-vis absorptivity of the Earth is not equal to the long wave IR emissivity. In addition, ice and clouds are highly reflective. You must also consider that water (the oceans) loses energy slower than solid surfaces and that a lot of solar energy is directly absorbed by the atmosphere. Q Science (talk) 23:41, 9 January 2010 (UTC)
- "The uv-vis absorptivity....", perhaps so but this is no reason for the frequently made assumption that "the Earth radiates like a black body in the infrared". A non-black body is a less efficient radiator than a black body because it has non-black properties i.e. it reflects, it is coloured, it is (partially) transparent, all of which take away from its "blackness", hence it has a lesser ability to radiate in total, in which ever part of the spectrum the radiating takes place, thus it is incapable of "radiating like a black body" i.e. with the equivalent efficiency of a black body. --Damorbel (talk) 12:51, 10 January 2010 (UTC)
- "like a blackbody" means that the total energy is proportional to the fourth power of the temperature AND that the spectrum is similar to the spectrum of a perfect black body. With that as a starting point, other parameters are defined to explain exactly how the object is NOT like a blackbody. Absorptivity and emissivity are simply two of those parameters.
- "... a blackbody" means that the total energy is..." This is quite insufficient. The relevant BB equ. is where, for a non-black body Є = 1- reflectance (or albedo if you will). Yes the spectrum may have a similar shape to a black body (don't forget that the BB spectrum at different temperatures has not only different T4 energy but also a different shape). All this means that a body that reflects (or transmits) some of the incident radiation i.e. it is not black cannot match a true BB in quantity of emitted radiation for a given temperature i.e. it will always be warmer than a measurement that assumes BB properties would indicate. This is a well known effect, see page 10 of [6] (it takes a long time to load). This is the nonsense behind the Earth's 255K equilibrium temperature and its supposed 33°C warming by the greenhouse effect .
- The assumption that the Earth can "emit in the infrared" with the same efficiency as a black body would mean that the material reflecting the Sun's radiation (i.e. the reason we can see the Blue Planet) is also able to radiate energy. It cannot do both, if it reflects radiation it cannot simultaneously absorb it, so where does this supposed emitted energy come from - nowhere? This is the Perpetual Motion aspect of the GHE.--Damorbel (talk) 07:28, 11 January 2010 (UTC)
- Different frequencies. The Earth does not emit blue light. It's blue light
emissivityemission is zero. However, the Earth is very good at absorbing blue light. It also reflects a lot of blue light. In the infrared (IR), the Earth emits about 85% of the number of photons that a blackbody would emit. (Some surfaces emit more.) The solar spectrum (at the top of the atmosphere - TOA) contains a lot of blue light but almost no IR photons. Almost all the IR photons at the TOA are emitted by the tops of the clouds. This is what weather satellites detect.
- Different frequencies. The Earth does not emit blue light. It's blue light
- Clarification: Emissivity refers to what would be emitted is the temperature is high enough. Emission refers to what is emitted at the current temperature. Q Science (talk) 17:11, 12 January 2010 (UTC)
- Your equation is not complete because it does not integrate over the complete frequency spectrum. The integration is absolutely critical. Q Science (talk) 08:55, 11 January 2010 (UTC)
- There have been many measurements of the energy from the surface of the Earth. Some are made from towers, others from aircraft at various altitudes, and a bunch from orbit. Those from short towers look a lot like standard blackbody spectra. As the measurements are made from higher up, they look more like a lot of different spectra added together. In fact, that is part of the basis of one of the alternate theories on how Greenhouse gases work. Q Science (talk) 04:02, 11 January 2010 (UTC)
- "water (the oceans) lose energy slower than solid surfaces" With respect, what do you mean by this? Water loses heat mainly by evaporation; a wet surface under radiation does not get hot until is dry, ja oder nein? --Damorbel (talk) 12:51, 10 January 2010 (UTC)
- You are absolutely correct, evaporation is more important than blackbody radiation. I was only referring to the radiative loses. Sorry for the confusion. Q Science (talk) 04:02, 11 January 2010 (UTC)
- The reference for that development is from "Planetary science: the science of planets around stars" by Cole and Woolfson. I don't have a copy of that book, do you happen to have a copy? PAR (talk) 03:40, 10 January 2010 (UTC)
- Sorry, no I don't have it (but it is available online). A search for the word "emissivity" indicates that it appears only once. I suggest that you find another source. (Perhaps Satellite thermal control for systems engineers, Robert D. Karam, page 158, table 6.1) In better books it explains that that both absorptivity and emissivity are functions of frequency. For many substances (like ice) the solar absorptivity is very low while the IR emissivity is fairly high. As a result, the average temperature is based on the ratio of a/e and that ratio is never equal to one (except for a black body). For instance, the absorptivity of white paint is about 0.17 and its emissivity is 0.86. Q Science (talk) 08:31, 10 January 2010 (UTC)
- Thanks, I found the online source, but the page that gives the development for the planetary temperature is not included. Also, the ratio of emission to absorption is also unity for "grey" bodies, in which the absorption and emission coefficients are constant with respect to wavelength. I understand that emission and absorption are functions of wavelength. I'm trying to understand your objection to the analysis given, however. To say that the earth reflects as a non-black body (because of the albedo, or reflectance) yet emits as a black body is a strong violation of Kirchoffs law. Are you saying that there is a strong violation? I mean, forget my analysis that assumes the Earth is a grey body, what I am saying is that the present analysis is a strong violation of Kirchoff's law. PAR (talk) 14:53, 10 January 2010 (UTC)
Ok, I get it. If you do everything as a function of frequency, then you get an average absorptance, averaged over the black body spectrum of the sun, and an average emissivity, averaged over a black body emission at Earth temperature and they are not necessarily equal. If is the sun-averaged absorptivity of the earth and is the earth-averaged emissivity, then the Earth temperature is
This is about the same as in the present development using where is albedo, or averaged reflectance. The problem now is the average emissivity, which was just assumed to be unity in the present development. Does anyone know by what logic we assume that the average emissivity of the Earth is unity, assuming no greenhouse effect? PAR (talk) 14:37, 11 January 2010 (UTC)
- Try the MODIS (Moderate Resolution Imaging Spectrometer) UCSB Emissivity Library. The area of interest is 3um to 65um. Unfortunately, the plots only go to 14 um. Q Science (talk) 18:24, 11 January 2010 (UTC)
Don't want to spoil your party but there is no way the reflecting Earth with its albedo can have an emissivity Є = 1, to do that the whole planet would have to behave like a black body, a body that absorbs all radiation that falls on it and reflects nothing. Q Science, you seem to believe it can, how?--Damorbel (talk) 22:00, 11 January 2010 (UTC)
- It might help if you look at the MODIS graphs before you comment on them. These are based on actual measurements, not theory. Q Science (talk) 22:58, 11 January 2010 (UTC)
Emissivity of 1 is not an assumption, nor an approximation, but just a reference condition that's used for what's called the "effective temperature". Dicklyon (talk) 22:21, 11 January 2010 (UTC)
- "just a reference condition"? The only reference condition with Є = 1 is a blackbody thus it is an assumption, an assumption that has no justification for a body that partially reflects incident radiation. Black bodies absorb all incident radiation thus they cannot reflect any, the Earth reflects radiation so any calculation that relies on Є = 1 will not give a thermodynamically consistent temperature i.e. it is off along the well trodden perpetual motion path. --Damorbel (talk) 07:59, 12 January 2010 (UTC)
- Damorbel - strictly speaking, you are right and the present article is wrong to say "If we assume the following that the Sun and the Earth both radiate as spherical black bodies". I think its a matter of the original author transcribing an argument, but not understanding what they were writing, because the equation presented is roughly correct. The earth reflects about 30% of the sunlight striking it. That's because the sun is at a very high temperature and it emits mostly in the visible and ultraviolet (VUV). So the earth is definitely not behaving like a black body in the VUV. The earth absorbs 70% of that sunlight and is warmed by it. It emits this same amount of energy according to its own temperature, not that of the sun, which means it emits in the infrared. In the infrared, the earth is a very good emitter, emitting almost the same as a perfect black body. So the "albedo" or reflectance is an average for the Earth in the VUV, and in the infrared, it has been assumed that the average emissivity of the Earth is 1. I will rewrite the argument hopefully to make this clear. PAR (talk) 15:04, 12 January 2010 (UTC)
Hi Dicklyon - the present development is looking for a definition of the greenhouse effect - the temperature increase due to the earth's atmosphere, so I think what we want is the emissivity of a no-atmosphere earth. Which, to QScience, I would ask, is it fair to base the emissivity on ice, since without an atmosphere there would be no ice or water? And to Damorbel, I am starting to understand QScience's statement that it is a function of frequency. The earth can be like a black body in some wavelengths and not in others. The albedo is about how it behaves in the visible frequency range, the black body is about how it behaves in the infrared frequency range. QScience is saying that because of ice and vegetation and stuff, the earth is like a black body in the infrared. PAR (talk) 02:20, 12 January 2010 (UTC)
- "The earth can be like a black body in some wavelengths and not..." No it can't, as I noted above if it reflects (or transmits) at any wavelength it is necessarilly non-black, just the same as if it reflected a little at all wavelengths (the "grey body" hypothesis). Just think about it, if it reflected (or transmitted) in the yellow and you told someone this bright yellow object was "a black body" you would get some rather strange looks. If it was a job interview maybe some"body" with different ideas gets the job. --Damorbel (talk) 07:59, 12 January 2010 (UTC)
- To see the Earth without ice, ocean, or atmosphere, look at the Moon. Q Science (talk) 04:08, 12 January 2010 (UTC)
- I cannot find a source that nails it down, but a number of sources suggest an average emissivity of 0.95, so saying its unity is not too far off. I will try to rewrite the present analysis a bit more clearly. PAR (talk) 05:55, 12 January 2010 (UTC)
- There was some confusion as to the "effective temperature" and the estimated temperature given present conditions. I also added an estimate of the temperature without atmosphere by inserting albedo and emissivity of the moon. PAR (talk) 16:48, 12 January 2010 (UTC)
Article changes
Please put back the section on computing the blackbody temperature of a planet. If you want to produce a major rewrite, you need to create a work page and develop it there. Making major changes in the article space is not going to work.
In addition, your statement
- Because of its high temperature, the sun emits almost entirely in the visible and ultraviolet (VUV) frequency range.
is not correct. The Sun emits much more IR radiation than the Earth. However, because the Sun is so far away, the amount of IR radiation at the top of the atmosphere is insignificant. This is because of "one over R squared" dimming and not what is emitted. Q Science (talk) 17:29, 12 January 2010 (UTC)
- Hi - sorry about the unintended deletion. Yes, "entirely" is not a good word to use. 90 percent of the sun's radiation is to the blue side of 1 micron. so, a "large majority" might be a better term. I meant it in relative terms, not absolute terms, certainly the sun emits more IR than the earth, but as a percentage of its total, its small. Also, all the changes that I made are things that I believe we agree on. PAR (talk) 18:07, 12 January 2010 (UTC)
- I still think that it is better to develop the new text in a work area. These are a few of the problems that need discussing, fixing, or clarification.
- The instruments I use refer to the solar spectrum as UV-Vis, not VUV which typically means vacuum ultraviolet.
- The solar spectrum measured in space is referred to as TOA (Top of Atmosphere). The effective temperature of the Sun is computed from that value, not the other way around.
- You dropped the abs and emt qualifiers (absorbed and emitted) from a number of variables. I think that this makes the formulas less clear.
- The Earth does not reflect "a fraction 1 − α of this energy"
- There are other issues, but these are enough for now. Q Science (talk) 21:18, 12 January 2010 (UTC)
- I fixed all of the above problems, plus a few typo's. (I did not use "TOA", but stressed that the solar radiation was that striking the top of the atmosphere rather than the Earth's surface). I think that it will not take a large number of iterations to get this right. PAR (talk) 21:58, 12 January 2010 (UTC)
Emissivity varies with wavelength. In the longwave (LW) the Earth (surface) is essentially "black" - ie, emissivity ~1 (actually ~0.997 if I recall correctly. Was that an average or minimum? Can't remember). In the shortwave (SW, i.e. visible) the emissivity, i.e. the (1-albedo), varies, as we can see William M. Connolley (talk) 22:11, 12 January 2010 (UTC)
- "In the longwave (LW) the Earth (surface) is essentially "black"...". As I noted above, this is illogical. Look at it this way, is a blue object "black" in the red part of the spectrum? Can you justify using the Є = 1 of a 100% black body in this case? --Damorbel (talk) 07:19, 13 January 2010 (UTC)
- It depends on what the definition of "black" is. With the red and blue, "black" is the absense of photons. You should interpret William's comment as saying "In the longwave (LW) the Earth (surface) emits photons with the same intensity and spectrum as a theoretical blackbody". In this context, "black" means radiating energy, not absorbing it. Think of the filament of an incandescent light bulb, it is a blackbody that gives off light. The surface of the Earth does the same thing, but, since the temperature is lower, your eyes can not see the photons. Q Science (talk) 08:11, 13 January 2010 (UTC)
- It depends on what the definition of "black...". This may be the difficulty. A black body in the thermal sense neither reflects nor transmits any incident radiation - at all. --Damorbel (talk) 09:22, 13 January 2010 (UTC)
- Right, but it does emit radiation. It emits its own, internally generated thermal radiation. For a body at room temperature, all that emission is in the infrared, so you cannot see it, so it looks black. But when it gets heated up, that thermal radiation moves into the visible. A hot black body glows red, but physicists still call it by the name "black body" because it doesn't reflect or transmit any radiation. Also, for non-black bodies, the emissivity can be different for different wavelengths. For any wavelength where the emissivity is one, physicists say it is "black" at that wavelength or you could say "it acts like a black body at that wavelength". By Kirchoff's law, it won't reflect or transmit any radiation at that wavelength. But any body that did not have its emissivity equal to one at every wavelength could not be called a black body. PAR (talk) 17:14, 13 January 2010 (UTC)
- "or you could say "it acts like a black body at that wavelength" I am afraid that just doesn't add up. In this case the formula for black body radiation just doesn't describe the radiated output. Look at it this way, suppose your non-black body emitted at a green band, in some circumstances it could be that the output had the intensity of a black body at that wavelength but it would not have the energy of a black body. Think further, green radiation doesn't have a temperature because it doesn't have a blackbody spectrum. Your green radiation could have the same energy as a black body but this would be extremely difficult to do, for starters you would have to produce narrow band radiation from heat with the same efficiency as a black body i.e. the maximum possible. --Damorbel (talk) 21:44, 13 January 2010 (UTC)
- Maybe a better way to say it is this - suppose you had a filter that only passed red light, like 600 nanometers. If you looked through that filter at a hot black body and some other body with the same shape and size and distance, with no outside light falling on them, and both at the same temperature, and they looked the same, same brightness, then that other body is black at that red wavelength. If you looked at them both through a green filter, and they looked different, then that other body is not black at that green wavelength. And that could happen. PAR (talk) 22:44, 13 January 2010 (UTC)
- "a hot black body and some other body..."; are they both at the same temperature? "And they looked the same", but there is "no outside light falling on them" so there is nothing for the non-black to reflect (or transmit). "And they looked different". Assuming they are at the same temp. etc., then the one that was non-black would seem cooler using an infrared thermometer (green filter) because its non-black property would make it a less efficient emitter, even though the two bodies were at the same (contact) thermometric temperature. (Have I understood your question?) --Damorbel (talk) 22:21, 14 January 2010 (UTC)
- Right - both at the same temperature, neither one having any light to reflect. But an infrared thermometer measures in some infrared wavelength band, and we are only looking at them thru a green and a red filter. An IR thermometer will of course give a correct reading for the black body, but who knows what the other one will read? We have not specified whether the other body is black or not in the infrared range of the IR thermometer, only that it is black in the wavelength range of the red filter, and yes, it will look dimmer thru the green filter and therefore is not black in the wavelength range of the green filter. PAR (talk) 23:03, 14 January 2010 (UTC)
- "only that it is black in the wavelength range of the red filter". I think the problem lies here. Do you mean that the emission strength of a particular waveband for a non-black body is the same as that of a black body? You are making very particular cases with filters etc. and "how things look", we are far from the general case of black and non-black, for example a non-black body can be (partially)transparent in the infrared i.e. only absorb in part of the spectrum, it might be completely transparent in which case it would look "black" in this waveband at any temperature. I'm sorry about my need for detail but if you are not happy about the general case and need to resort to filters and "how things look" I suggest you should look carefully at you qualifications for rewriting the article. --Damorbel (talk) 07:31, 15 January 2010 (UTC)
Temperature relation between a planet and its star - Move to Effective temperature?
This development is partially or fully duplicated, more or less, in a number of articles,
- Black body#Temperature relation between a planet and its star
- Effective temperature#Planet
- Greenhouse effect
- Planck's law
- Idealized greenhouse model
I wonder if the present development would better be shortenend, and then pointed to the Effective temperature#Planet article? PAR (talk) 11:34, 16 January 2010 (UTC)
- Effective temperature#Planet is pretty short. It is also a bit different. I would not support that move. Q Science (talk) 18:47, 17 January 2010 (UTC)
- I would like to combine the two, so nothing is lost. I think in the present article we have gone beyond a simple black body. I will work on the effective temperature article to incorporate the present development, then see if its worth pointing this article to it and reducing the amount in this article. PAR (talk) 16:22, 18 January 2010 (UTC)
This seems to be a contradiction
First I read this:
The total surface area of an adult is about 2 m², and the mid- and far-infrared emissivity of skin and most clothing is near unity, as it is for most nonmetallic surfaces.[11][12] Skin temperature is about 33°C,[13] but clothing reduces the surface temperature to about 28 °C when the ambient temperature is 20 °C.[14] Hence, the net radiative heat loss is about 100 watts.
Then I read this:
Due to the rapid fall-off of emitted photons with decreasing energy, a black body at room temperature (300 K) with 1 m2 of surface area emits a visible photon every thousand years or so, which is negligible for most purposes.
Okey so we have gone from 2 square meters to one, and from 28 degrees Celsius to room temperature, but still; only one photon emitted every thousand year or so!?!? How can there possibly be such an extreme difference between the two examples, please someone explain this to me! --Nabo0o (talk) 20:06, 8 May 2010 (UTC)
- Lol, okey it seems I didn't notice the "visible" part of the sentence ;D Sorry for the disturbance --Nabo0o (talk) 18:49, 6 July 2010 (UTC)
Wien's displacement law
The equation that was there before compared the maximum wavelength per unit wavelength with the maximum wavelength per unit frequency. Since this is useful information, and since many people don't know that there is a significant difference, I have restored that equation. Q Science (talk) 17:22, 1 July 2010 (UTC)
- The maximum frequency is a more natural statement than the maximum wavelength for that situation. ScienceApologist (talk) 19:55, 1 July 2010 (UTC)
- No it is not. This is a point of considerable confusion and the difference needs to be explicitly made. The wavelength of maximum power is actually different if the Plank blackbody equation is integrated with respect to wavelength or with respect to frequency. In addition, most people know that the wavelength of green light is 550 nm, but how many people know the frequency? In fact, at visible wavelengths (and above), almost no one uses frequency. Instead, they all use wavelength. (Well, at very high frequencies they use electron volts.) Q Science (talk) 22:16, 1 July 2010 (UTC)
- I would agree with Q Science that wavelength is used much more often for UV-visible-IR range (i.e. above GHz), especially in the visible (and frequency is perhaps more common at GHZ and below). Materialscientist (talk) 22:21, 1 July 2010 (UTC)
- Since Wein's Displacement Law applies for all wavelengths/frequencies, it is inappropriate for us to deprecate according to convention around the visible wavelengths. The cleanest approach is to work entirely in one spectral formalism or the other. Translation occurs by means of unit conversion and careful attention to Jacobians, but it is silly to list the peak wavelength when if you plotted specific intensity versus wavelength it would either peak at the incorrect wavelength or be improperly normalized. Sticking to one unit system avoids this confusion that elementary students of radiative transfer make all the time. ScienceApologist (talk) 23:35, 1 July 2010 (UTC)
- I would agree with Q Science that wavelength is used much more often for UV-visible-IR range (i.e. above GHz), especially in the visible (and frequency is perhaps more common at GHZ and below). Materialscientist (talk) 22:21, 1 July 2010 (UTC)
- No it is not. This is a point of considerable confusion and the difference needs to be explicitly made. The wavelength of maximum power is actually different if the Plank blackbody equation is integrated with respect to wavelength or with respect to frequency. In addition, most people know that the wavelength of green light is 550 nm, but how many people know the frequency? In fact, at visible wavelengths (and above), almost no one uses frequency. Instead, they all use wavelength. (Well, at very high frequencies they use electron volts.) Q Science (talk) 22:16, 1 July 2010 (UTC)
- Please clarify what you mean by "the incorrect wavelength". And could you please explain how Jacobians are used in this case. Q Science (talk) 05:23, 2 July 2010 (UTC)
If you plot specific intensity per unit frequency versus wavelength, the integral is not the specific intensity. If you plot specific intensity per unit wavelength versus wavelength, the integral is correct, but the peak is according to the first rather than second equation of interest. A Jacobian is used in calculus when you translate between one coordinate system and another (in this case, frequency space and wavelength space). ScienceApologist (talk) 13:59, 2 July 2010 (UTC)
- According to hyperphysics, there are 4 different correct peak wavelengths, depending on what instrument is used to measure the spectra or which method is used to plot it. There is even a (very good) calculator. For instance, 500 nm (green) is associated with a solar temperature of 5,269 K. However, if the same spectra was plotted verses frequency in a way that both spectra would have the same integral, then the peak would be at 968 nm, in the infrared. As I understand it, this does not make one peak right and the other wrong, they are just different. The equation you deleted made this obvious and that is the reason I think it should be restored. Q Science (talk) 14:41, 2 July 2010 (UTC)
- The equation I deleted is identical to the one I put up. Almost everything you wrote is more=or-less correct, but mathematically, the way I present it is cleaner. ScienceApologist (talk) 16:21, 2 July 2010 (UTC)
- I don't agree. If it was identical, then there was no reason for you to delete it, twice, so far. Why don't we compromise and leave both equations. Q Science (talk) 20:22, 2 July 2010 (UTC)
- They are identical in the same way that xy = 3 and x = 3/y are identical. The reason I deleted it is because specific intensity per frequency should yield a frequency rather than a wavelength. ScienceApologist (talk) 13:22, 3 July 2010 (UTC)
Error
Article says "at 6000 K it looks white". This is not true, it's the temperature of the sun's surface, which to us looks slightly yellow, because what we consider white illumination has been filtered by the atmosphere. And this is why sRGB normally uses D65, which is (non-black-body) 6504 K, and this in turn is why the image blends into the white background around 6500 K. —Preceding unsigned comment added by 82.139.87.74 (talk) 05:34, 8 August 2010 (UTC)
- The article is discussing an oven, not the Sun. The perceived color of the Sun does not match its blackbody temperature because blue light is scattered by the atmosphere more than red. On the other hand, I suggest that you to add a short section discussing filters with a link to a more in depth discussion. Q Science (talk) 07:19, 27 August 2010 (UTC)
Major edits
GianniG46 (talk) has marked sections as needing citations. Since, in my opinion, those sections were wrong, I deleted them. I also deleted the following
- Since only matter has a temperature
- the study of black-body radiation reveals how continuous fields can have a temperature,
- Shiny surfaces effect the amount of energy emitted, but not the color
- so long as the oven is not too shiny, the color ... depends on the temperature
I also deleted most (not all) of the unreferenced material added 18 August 2009 by Chjoaygame There are many other problems with this section. Please help make it right. Q Science (talk) 19:32, 17 October 2010 (UTC)
- I removed in turn the words in italics from the sentence "provided strictly that the cavity contains some perfectly black material body and is in radiative equilibrium.[6]" which is apparently cited (but the citation is not specific, is a text on statistical mechanics". I, too, think there is still more to do in this section, to put the material into a logical sequence, add some explanations, and remove the many redundancies. --GianniG46 (talk) 10:59, 18 October 2010 (UTC)
Please explain "per unit volume"
Dicklyon, please explain your edit. When we plot the blackbody radiation from the Sun, we typically plot energy per square meter, not per unit volume. And "unit volume" of what? The emitting surface? The detector area? What am I missing? Q Science (talk) 20:04, 20 October 2010 (UTC)
- Indeed, the plot with the "arbitrary" scale gives the spectrum of blackbody radiation, but doesn't say anything about the energy density in equilibrium. The "per volume" applies in a cavity in equilibrium. See this book for example, which shows an explicit division of energy by volume. This is the more explicit function that the original editor must have been referring to; I don't know if "black body curve" is the best thing to call it. Dicklyon (talk) 20:10, 20 October 2010 (UTC)
- It can also be expressed as power per unit area, without an arbitrary factor (unlike the power per unit area in solar radiation at the Earth, which has a factor that depends on lots of things besides the blackbody temperature); but plain energy, or plain power, won't work.
- Well, the plots in that book are of P(v,T)dv (radiative flux) which has units Power/area/steradian. Perhaps this article should be changed to reflect that since that is what is plotted in the image. I don't see how a "per volume" definition is useful here without considerable explanation for the casual reader.
- Also, why did you remove the word distribution in the first part of my edit? I added that because "white" is not the color of any specific wavelength. Q Science (talk) 20:52, 20 October 2010 (UTC)
- I reverted your edit because it changed correct dimensions to incorrect dimensions. You can rework it. Dicklyon (talk) 21:24, 20 October 2010 (UTC)
Of course the dimensions are both correct: power per unit area and energy per unit volume differ by a velocity (of light): the first is more usual in BB radiation curves, the second in speaking of equilibrium radiation. So the first would be preferable, were it not that the paragraph is speaking of cavities. I think choosing one or another is not so crucial.
The same perhaps can be told of a previous edit of mine of the same sentence. I have put first frequency units (wich are more usual in Planck's law and physically much more "sound"), then those in wavelength units. But the graph reported in the first figure of the article is in wavelength units, which are in use mainly because of their practicity in optics and spectrometry. --GianniG46 (talk) 08:19, 21 October 2010 (UTC)
- I've reworked the paragraph again to clarify that what makes the oven a good black body is exactly its characteristic of absorbing all the light that enters. That's why it's more often described as a cavity with a small opening; "oven" may falsely give the impression of a big opening, like a kitchen oven. Dicklyon (talk) 20:48, 21 October 2010 (UTC)
PAR's edit
I reverted PAR's edit, as the sentence I quoted in my edit summary didn't seem to make sense. But I also disagree with his points in his edit summary. He says "color is not a measure of the wavelength distribution". Isn't it? I thought that was exactly what color was; or if not "a measure of" then at least "mediated by". That's why we have red hot, blue hot, etc. Admittedly, it's not a complete characterization of the wavelength distribution, as many distributions look red and many look bluish-white, but over a pretty good range, the color temperature is related to the color that characterizes the distribution of black-body spectra over a good range of temperatures. And he said "an oven is not a black body". I think I disagree, if by oven we mean a cavity with a small hole in it. It's pretty exactly a black body, at least in the limits usually considered, where even the material it's made of doesn't matter. There's probably a more gentle way to patch the paragraph if it needs clarification. Dicklyon (talk) 05:55, 21 October 2010 (UTC)




























