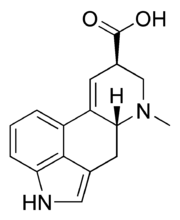
Lysergic acid
| |

| |
| Names | |
|---|---|
| IUPAC name
7-Methyl- 4,6,6a,7,8,9- hexahydro- indolo [4,3-fg] quinoline- 9-carboxylic acid
| |
| Other names
6-Methyl- 9,10- didehydroergoline- 8-carboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.302 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H16N2O2 | |
| Molar mass | 268.316 g·mol−1 |
| Melting point | 238 - 240 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lysergic acid, also known as D-lysergic acid and (+)-lysergic acid, is a precursor for a wide range of ergoline alkaloids that are produced by the ergot fungus and some plants. Amides of lysergic acid, lysergamides, are widely used as pharmaceuticals and as psychedelic drugs (LSD). Lysergic acid received its name as it was a product of the lysis of various ergot alkaloids [1].
Synthesis
Lysergic acid is generally produced by hydrolysis[2] of natural lysergamides, but can also be synthesized in the laboratory by a complex total synthesis for example by Woodward's team in 1956 [3]. An enantioselective total synthesis based on a palladium catalyzed domino cyclization reaction has been described in 2011 by Fujii and Ohno.[4] Lysergic acid monohydrate crystallizes in very thin hexagonal leaflets when recrystallized from water. Lysergic acid monohydrate, when dried (140 °C at 2 mmHg or 270 Pa) forms anhydrous lysergic acid. The biosynthetic route is based on the alkylation of the amino acid Tryptophan with dimethylallyl diphosphate (isoprene derived from 3R-mevalonic acid) giving 4-dimethylallyl-L-tryptophan which is N-methylated with S-adenosyl-L-methionine. Oxidative ring closure followed by decarboxylation, reduction, cyclization, oxidation, and allylic isomerization yields D-(+)-lysergic acid.[1]
Isomers
Lysergic acid is a chiral compound with two stereocenters. The isomer with inverted configuration at carbon atom 8 close to the carboxy group is called isolysergic acid. Inversion at carbon 5 close to the nitrogen atom leads to L-lysergic acid and L-isolysergic acid, respectively. Lysergic acid is listed as a Table I precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.[5]

See also
- Lysergic acid diethylamide (also known as LSD/Acid)
- Lysergic acid amide (LSA/Ergine)
- Ergoline
- Lysergamides
References
- ^ a b Schiff PL (2006 Oct 15). "Ergot and its alkaloids". Am J Pharm Educ. 70 (5): 98. PMID 17149427.
{{cite journal}}: Check date values in:|date=(help) - ^ Martínková L, Kren V, Cvak L, Ovesná M, Prepechalová I (2001 Nov 17). "Hydrolysis of lysergamide to lysergic acid by Rhodococcus equi A4". J Biotechnol. 84 (1): 63–6.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ Edmund C. Kornfeld, E.J. Fornefeld, G. Bruce Kline, Marjorie J. Mann, Dwight E. Morrison, Reuben G. Jones and R.B. Woodward (1956). "The Total Synthesis of Lysergic Acid". Journal of the American Chemical Society. 78: 3087–3114.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ S. Inuki, A. Iwata, S. Oishi, N. Fujii and H. Ohno, J. Org. Chem. 2011, 76 (7), pp 2072–2083, doi:10.1021/jo102388e.
- ^ List of Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances Under International Control, International Narcotics Control Board
