Exocrine pancreatic insufficiency
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
| Exocrine pancreatic insufficiency | |
|---|---|
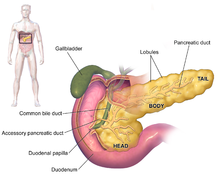 | |
| Anatomy of the pancreas | |
| Specialty | Endocrinology, gastroenterology |
| Causes | Pancreatitis |
Exocrine pancreatic insufficiency (EPI) is the inability to properly digest food due to a lack of digestive enzymes made by the pancreas. EPI is found in humans afflicted with cystic fibrosis and Shwachman–Diamond syndrome, and is common in dogs. EPI is caused by a progressive loss of the pancreatic cells that make digestive enzymes; loss of digestive enzymes leads to maldigestion and malabsorption of nutrients from normal digestive processes.
Chronic pancreatitis is the most common cause of EPI in humans and cats. In dogs, the most common cause is pancreatic acinar atrophy, arising as a result of genetic conditions, a blocked pancreatic duct, or prior infection.
The exocrine pancreas is a portion of this organ that contains clusters of ducts (acini) producing bicarbonate anion, a mild alkali, as well as an array of digestive enzymes that together empty by way of the interlobular and main pancreatic ducts into the duodenum (upper small intestine).[1] The hormones cholecystokinin and secretin secreted by the stomach and duodenum in response to distension and the presence of food in turn stimulate the production of digestive enzymes by the exocrine pancreas.[2] The alkalization of the duodenum neutralizes the acidic chyme produced by the stomach that is passing into it; the digestive enzymes serve to catalyze the breakdown of complex foodstuffs into smaller molecules for absorption and integration into metabolic pathways.[2] The enzymes include proteases (trypsinogen and chymotrypsinogen), hydrolytic enzymes that cleave lipids (the lipases phospholipase A2 and lysophospholipase, and cholesterol esterase), and amylase to digest starches. EPI results from progressive failure in the exocrine function of the pancreas to provide its digestive enzymes, often in response to a genetic condition or other disease state, resulting in the inability of the animal involved to properly digest food.[citation needed]
Signs and symptoms
Loss of pancreatic enzymes leads to maldigestion and malabsorption, which may in turn lead to:[citation needed]
- anemia (Vitamin B12, iron, folate deficiency)
- bleeding disorders (Vitamin K malabsorption)
- edema (hypoalbuminemia)
- fatigue
- flatulence and abdominal distention (bacterial fermentation of unabsorbed food)
- hypocalcemia
- metabolic bone disease (Vitamin D deficiency)
- neurologic manifestation
- steatorrhea
- weight loss
Causes
In humans, the most common causes of EPI are chronic pancreatitis and cystic fibrosis, the former a longstanding inflammation of the pancreas altering the organ's normal structure and function that can arise as a result of malnutrition, heredity, or (in the Western world especially), behaviour (alcohol use, smoking),[citation needed][3] and the latter a recessive hereditary disease most common in Europeans and Ashkenazi Jews where the molecular culprit is an altered, CFTR-encoded chloride channel.[citation needed] According to WebMD, "Crohn's disease and celiac disease can also lead to EPI in some people".[4] In children, another common cause is Shwachman-Bodian-Diamond syndrome, a rare autosomal recessive genetic disorder resulting from mutation in the SBDS gene.[citation needed]
Diagnosis
The three main tests used in considering a diagnosis of EPI are: fecal elastase test, fecal fat test, and a direct pancreatic function test. [5] The latter is a limitedly used test that assesses exocrine function in the pancreas by inserting a tube into the small intestine to collect pancreatic secretions.
Treatment
EPI is often treated with pancreatic enzyme replacement products (PERPs) such as pancrelipase, that are used to break down fats (via a lipase), proteins (via a protease), and carbohydrates (via amylase) into units that can be digested.[6] Pancrelipase is typically porcine derived and requires large doses.
Other animals
Causes and pathogenesis
Chronic pancreatitis is the most common cause of EPI in cats.[citation needed] In dogs, where the condition has been deemed common,[citation needed] the usual cause is by pancreatic acinar atrophy, arising as a result of genetic conditions, a blocked pancreatic duct, or prior infection.[7]
In dogs, EPI is most common in young German Shepherds, and in Finland Rough Collies,[8] and is inherited.[9] In German Shepherds, the method of inheritance is through an autosomal recessive gene.[10] In these two breeds, at least, the cause appears to be immune-mediated as a sequela to lymphocytic pancreatitis.[11] The German Shepherd makes up about two-thirds of cases seen with EPI.[12] Other breeds reported to be predisposed to EPI include terrier breeds, Cavalier King Charles Spaniels, Chow Chows,[9] and Picardy Shepherds.
Symptoms
In animals, signs of EPI are not present until 85 to 90 percent of the pancreas is unable to secrete its enzymes.[7] In dogs, symptoms include weight loss, poor hair coat, flatulence, increased appetite, coprophagia, and diarrhea. Feces are often yellow-gray in color with an oily texture. There are many concurrent diseases that mimic EPI, and severe pancreatitis is one that if allowed to continue unabated can lead to EPI.[citation needed]
Diagnosis and treatment
The most reliable test for EPI in dogs and cats is serum trypsin-like immunoreactivity (TLI);[13] a low value indicates EPI. Fecal elastase levels may also be used for diagnosis in dogs.[14]
In dogs, the best treatment is to supplement the animals' food with dried pancreatic extracts. There are commercial preparations available, but chopped bovine pancreas from the butcher can also be used. (Pork pancreas should not be used because of the rare transmission of pseudorabies).[15] Symptoms usually improve within a few days, but lifelong treatment is required to manage the condition. A rare side-effect of use of dried pancreatic extracts is oral ulceration and bleeding.[16]
Because of malabsorption, serum levels of cyanocobalamin (vitamin B12) and tocopherol (vitamin E) may be low. These may be supplemented, although since cyanocobalamin contains the toxic chemical cyanide, dogs that have serious cobalamin issues should instead be treated with hydroxocobalamin or methylcobalamin.[citation needed]
Cyanocobalamin deficiency is very common in cats with EPI because about 99 percent of intrinsic factor (which is required for cyanocobalamin absorption from the intestine) is secreted by the pancreas. In dogs, this figure is about 90 percent, and only about 50 percent of dogs have this deficiency.[15]
Cats may suffer from Vitamin K deficiencies. If there is bacterial overgrowth in the intestine, antibiotics should be used, especially if treatment is not working.
In dogs failing to gain weight or continuing to show symptoms, modifying the diet to make it low-fiber and highly digestible may help. Despite previous belief that low-fat diets are beneficial in dogs with EPI, more recent studies have shown that a high-fat diet may increase absorption of nutrients and better manage the disease.[17] However, it has been shown that different dogs respond to different dietary modifications, so the best diet must be determined on a case-by-case basis.[18]
One possible sequela, volvulus (mesenteric torsion), is a rare consequence of EPI in dogs.[7]
References
- ^ Pandol, Stephen J. (2010-01-01). Anatomy. Morgan & Claypool Life Sciences.
- ^ a b Pandol, Stephen J. (2010-01-01). Regulation of Whole-Organ Pancreatic Secretion. Morgan & Claypool Life Sciences.
- ^ Yadav, Dhiraj (2009-06-08). "Alcohol Consumption, Cigarette Smoking, and the Risk of Recurrent Acute and Chronic Pancreatitis". Arch Intern Med. 169 (11): 1035–45. doi:10.1001/archinternmed.2009.125. ISSN 0003-9926. PMC 6785300. PMID 19506173.
- ^ Ambardekar, Nayana MD (Reviewer) (December 12, 2017). "Exocrine Pancreatic Insufficiency". WebMD.
- ^ Domínguez-Muñoz, JE (2011). "Pancreatic insufficiency: diagnosis and treatment". J Gastroenterol Hepatol. 26 (Supplement s2): 12–16. doi:10.1111/j.1440-1746.2010.06600.x. PMID 21323992.
- ^ "FDA rulemaking history of OTC EPI drug products". Fda.gov. Retrieved 2011-11-08.
- ^ a b c Ettinger, Stephen J.; Feldman, Edward C. (1995). Textbook of Veterinary Internal Medicine (4th ed.). W.B. Saunders Company. ISBN 0-7216-6795-3.
- ^ "Exocrine pancreatic insufficiency in dogs". Vet Clin N Am Small Animal Pract. 33 (5): 1165–79, viii–ix. 2003. doi:10.1016/S0195-5616(03)00057-3. PMID 14552166.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b Hall, Edward J. (2003). "Exocrine Pancreatic Insufficiency". Proceedings of the 28th World Congress of the World Small Animal Veterinary Association. Retrieved 2007-02-24.
- ^ "Linkage analysis and gene expression profile of pancreatic acinar atrophy in the German Shepherd Dog". Mamm Genome. 16 (12): 955–62. 2005. doi:10.1007/s00335-005-0076-1. PMID 16341675. S2CID 33039280.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Exocrine pancreatic atrophy in German Shepherd Dogs and Rough-coated Collies: an end result of lymphocytic pancreatitis". Vet Pathol. 36 (6): 530–41. 1999. doi:10.1354/vp.36-6-530. PMID 10568434. S2CID 31368693.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Canine exocrine pancreatic insufficiency treated with porcine pancreatic extract". J Vet Sci. 6 (3): 263–6. 2005. doi:10.4142/jvs.2005.6.3.263. PMID 16131834.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Serum lipase activities and pancreatic lipase immunoreactivity concentrations in dogs with exocrine pancreatic insufficiency". Am J Vet Res. 67 (1): 84–7. 2006. doi:10.2460/ajvr.67.1.84. PMID 16426216.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Rallis, Timoleon S.; Adamama-Moraitou, K. (2004). "Exocrine Pancreatic Insufficiency in Dogs and Cats: An Update". Proceedings of the 29th World Congress of the World Small Animal Veterinary Association. Retrieved 2007-02-24.
- ^ a b "Exocrine Pancreatic Insufficiency". The Merck Veterinary Manual. 2006. Retrieved 2007-02-24.
- ^ Snead E (2006). "Oral ulceration and bleeding associated with pancreatic enzyme supplementation in a German shepherd with pancreatic acinar atrophy". Can Vet J. 47 (6): 579–82. PMC 1461413. PMID 16808232.
- ^ Biourge V, Fontaine J (2004). "Exocrine pancreatic insufficiency and adverse reaction to food in dogs: a positive response to a high-fat, soy isolate hydrolysate-based diet". J Nutr. 134 (8 Suppl): 2166S–2168S. doi:10.1093/jn/134.8.2166S. PMID 15284428.
- ^ "Effects of diet on clinical signs of exocrine pancreatic insufficiency in dogs". J Am Vet Med Assoc. 228 (2): 225–9. 2006. doi:10.2460/javma.228.2.225. PMID 16426193.
{{cite journal}}: Unknown parameter|authors=ignored (help)

