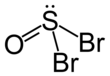
Thionyl bromide
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Thionyl bromide
| |||
| Other names
Sulfur oxy dibromide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.007.332 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SOBr2 | |||
| Molar mass | 207.87 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 2.688 g/mL, liquid | ||
| Melting point | −52 °C (−62 °F; 221 K) | ||
| Boiling point | 68 °C (154 °F; 341 K) at 40 mmHg | ||
| decomposes | |||
| Solubility | reacts in HBr, acetone, and alcohol soluble in benzene, toluene, ether | ||
| Structure | |||
| trigonal pyramidal | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Readily decomposes in air to toxic gases | ||
| Flash point | Non-flammable | ||
| Safety data sheet (SDS) | "External MSDS" | ||
| Related compounds | |||
Related compounds
|
SOCl2, SeOCl2; | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Thionyl bromide is the chemical compound SOBr2. It is less stable and less widely used than its chloride analogue, thionyl chloride. It is prepared by the action of hydrogen bromide on thionyl chloride, a characteristic reaction where a stronger acid is converted to a weaker acid:
- SOCl2 + 2 HBr → SOBr2 + 2 HCl
Thionyl bromide will convert alcohols to alkyl bromides and can be used for brominations of certain α,β-unsaturated carbonyl compounds.
Safety
SOBr2 hydrolyzes readily in air to release dangerous fumes of sulfur dioxide and hydrogen bromide.
- SOBr2 + H2O → SO2 + 2 HBr
References
Mundy, B. P. (2004). "Thionyl Bromide". In Paquette, E. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rt098.
Wikimedia Commons has media related to Thionyl bromide.