Presatovir
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Presatovir |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
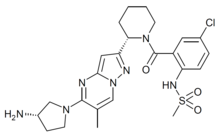
| Formula | C24H30ClN7O3S |
| Molar mass | 532.1 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Presatovir (GS-5806) is an antiviral drug which was developed as a treatment for respiratory syncytial virus. It acts as a fusion inhibitor, and has shown promising results in Phase II clinical trials.[1][2]
See also
References
- ^ Marty FM, Chemaly RF, Mullane KM, Lee DG, Hirsch HH, Small CB, et al. (December 2019). "A Phase 2b, Randomized, Double-blind, Placebo-Controlled Multicenter Study Evaluating Antiviral Effects, Pharmacokinetics, Safety, and Tolerability of Presatovir in Hematopoietic Cell Transplant Recipients with Respiratory Syncytial Virus (RSV) Infection of the Lower Respiratory Tract". Clinical Infectious Diseases. doi:10.1093/cid/ciz1167. PMID 31915807.
- ^ Stray K, Perron M, Porter DP, Anderson F, Lewis SA, Perry J, et al. (January 2020). "Drug resistance assessment following administration of RSV fusion inhibitor presatovir to participants experimentally infected with respiratory syncytial virus". The Journal of Infectious Diseases. doi:10.1093/infdis/jiaa028. PMID 31971597.
