Odalasvir
Appearance
(Redirected from ACH-3102)
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
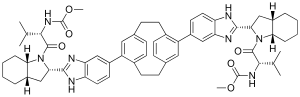
| Formula | C60H72N8O6 |
| Molar mass | 1001.286 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Odalasvir (INN,[1] previously known as ACH-3102)[2][3] is an investigational new drug in development for the treatment of hepatitis C.[4] It is an NS5A inhibitor.[5] The NS5A protein serves multiple functions at various stages of the viral life cycle, including viral replication. NS5A also plays a role in the development of interferon-resistance, a common cause of treatment failure.[6][7][8] It is under development by Achillion Pharmaceuticals.[citation needed]
See also
[edit]References
[edit]- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). RECOMMENDED International Nonproprietary Names: List 73" (PDF). who.int. World Health Organization. p. 38. Retrieved 5 December 2015.
- ^ "Achillion Has Discovered and Developed a Comprehensive Portfolio of Antivirals for the Treatment of Hepatitis C". Achillion Pharmaceuticals. Retrieved 15 November 2015.
- ^ "Odalasvir". ChemIDplus.
- ^ Walker T (December 2015). "Watch list 2016: top therapeutic areas: experts say you should follow these 6 therapeutic areas". Managed Healthcare Executive: 47. Retrieved March 12, 2016.
- ^ Nakamoto S, Kanda T, Wu S, Shirasawa H, Yokosuka O (March 2014). "Hepatitis C virus NS5A inhibitors and drug resistance mutations". World Journal of Gastroenterology. 20 (11): 2902–12. doi:10.3748/wjg.v20.i11.2902. PMC 3961994. PMID 24659881.
- ^ "ACH-3102 resources". Achillion Pharmaceuticals. Winter 2011. Retrieved 1 May 2012.
- ^ "Achillion gets FDA incentives for hepatitis C drug". Associated Press. 15 May 2012. Archived from the original on May 22, 2012.
- ^ Levin J (18–22 April 2012). Preclinical characteristics of ACH-3102. 47th Annual Meeting. Barcelona, Spain: European Association for the Study of the Liver.
