Chlorophyll
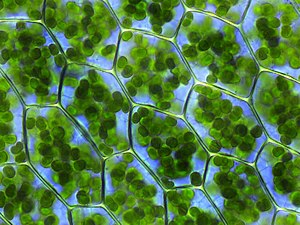
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants.[2] Its name is derived from the Greek words χλωρός (khloros, "pale green") and φύλλον (phyllon, "leaf").[3] Chlorophyll allows plants to absorb energy from light.
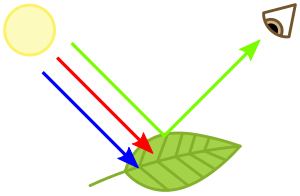
Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion.[4] Conversely, it is a poor absorber of green and near-green portions of the spectrum. Hence chlorophyll-containing tissues appear green because green light, diffusively reflected by structures like cell walls, is less absorbed.[1] Two types of chlorophyll exist in the photosystems of green plants: chlorophyll a and b.[5]
History
[edit]Chlorophyll was first isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817.[6] The presence of magnesium in chlorophyll was discovered in 1906,[7] and was the first detection of that element in living tissue.[8]
After initial work done by German chemist Richard Willstätter spanning from 1905 to 1915, the general structure of chlorophyll a was elucidated by Hans Fischer in 1940. By 1960, when most of the stereochemistry of chlorophyll a was known, Robert Burns Woodward published a total synthesis of the molecule.[8][9] In 1967, the last remaining stereochemical elucidation was completed by Ian Fleming,[10] and in 1990 Woodward and co-authors published an updated synthesis.[11] Chlorophyll f was announced to be present in cyanobacteria and other oxygenic microorganisms that form stromatolites in 2010;[12][13] a molecular formula of C55H70O6N4Mg and a structure of (2-formyl)-chlorophyll a were deduced based on NMR, optical and mass spectra.[14]
Photosynthesis
[edit]
Chlorophyll is vital for photosynthesis, which allows plants to absorb energy from light.[15]
Chlorophyll molecules are arranged in and around photosystems that are embedded in the thylakoid membranes of chloroplasts.[16] In these complexes, chlorophyll serves three functions:
- The function of the vast majority of chlorophyll (up to several hundred molecules per photosystem) is to absorb light.
- Having done so, these same centers execute their second function: The transfer of that energy by resonance energy transfer to a specific chlorophyll pair in the reaction center of the photosystems.
- This specific pair performs the final function of chlorophylls: Charge separation, which produces the unbound protons (H+) and electrons (e−) that separately propel biosynthesis.
The two currently accepted photosystem units are photosystem I and photosystem II, which have their own distinct reaction centres, named P700 and P680, respectively. These centres are named after the wavelength (in nanometers) of their red-peak absorption maximum. The identity, function and spectral properties of the types of chlorophyll in each photosystem are distinct and determined by each other and the protein structure surrounding them.
The function of the reaction center of chlorophyll is to absorb light energy and transfer it to other parts of the photosystem. The absorbed energy of the photon is transferred to an electron in a process called charge separation. The removal of the electron from the chlorophyll is an oxidation reaction. The chlorophyll donates the high energy electron to a series of molecular intermediates called an electron transport chain. The charged reaction center of chlorophyll (P680+) is then reduced back to its ground state by accepting an electron stripped from water. The electron that reduces P680+ ultimately comes from the oxidation of water into O2 and H+ through several intermediates. This reaction is how photosynthetic organisms such as plants produce O2 gas, and is the source for practically all the O2 in Earth's atmosphere. Photosystem I typically works in series with Photosystem II; thus the P700+ of Photosystem I is usually reduced as it accepts the electron, via many intermediates in the thylakoid membrane, by electrons coming, ultimately, from Photosystem II. Electron transfer reactions in the thylakoid membranes are complex, however, and the source of electrons used to reduce P700+ can vary.
The electron flow produced by the reaction center chlorophyll pigments is used to pump H+ ions across the thylakoid membrane, setting up a proton-motive force a chemiosmotic potential used mainly in the production of ATP (stored chemical energy) or to reduce NADP+ to NADPH. NADPH is a universal agent used to reduce CO2 into sugars as well as other biosynthetic reactions.
Reaction center chlorophyll–protein complexes are capable of directly absorbing light and performing charge separation events without the assistance of other chlorophyll pigments, but the probability of that happening under a given light intensity is small. Thus, the other chlorophylls in the photosystem and antenna pigment proteins all cooperatively absorb and funnel light energy to the reaction center. Besides chlorophyll a, there are other pigments, called accessory pigments, which occur in these pigment–protein antenna complexes.
Chemical structure
[edit]
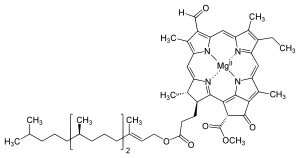
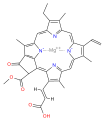
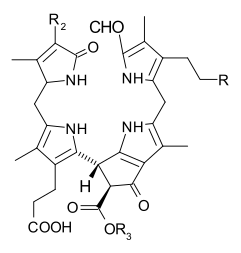
Several chlorophylls are known. All are defined as derivatives of the parent chlorin by the presence of a fifth, ketone-containing ring beyond the four pyrrole-like rings. Most chlorophylls are classified as chlorins, which are reduced relatives of porphyrins (found in hemoglobin). They share a common biosynthetic pathway with porphyrins, including the precursor uroporphyrinogen III. Unlike hemes, which contain iron bound to the N4 center, most chlorophylls bind magnesium. The axial ligands attached to the Mg2+ center are often omitted for clarity. Appended to the chlorin ring are various side chains, usually including a long phytyl chain (C20H39O). The most widely distributed form in terrestrial plants is chlorophyll a. The only difference between chlorophyll a and chlorophyll b is that the former has a methyl group where the latter has a formyl group. This difference causes a considerable difference in the absorption spectrum, allowing plants to absorb a greater portion of visible light.
The structures of chlorophylls are summarized below:[17][18]
| Chlorophyll a | Chlorophyll b | Chlorophyll c1 | Chlorophyll c2 | Chlorophyll d | Chlorophyll f[14] | |
|---|---|---|---|---|---|---|
| Molecular formula | C55H72O5N4Mg | C55H70O6N4Mg | C35H30O5N4Mg | C35H28O5N4Mg | C54H70O6N4Mg | C55H70O6N4Mg |
| C2 group | −CH3 | −CH3 | −CH3 | −CH3 | −CH3 | −CHO |
| C3 group | −CH=CH2 | −CH=CH2 | −CH=CH2 | −CH=CH2 | −CHO | −CH=CH2 |
| C7 group | −CH3 | −CHO | −CH3 | −CH3 | −CH3 | −CH3 |
| C8 group | −CH2CH3 | −CH2CH3 | −CH2CH3 | −CH=CH2 | −CH2CH3 | −CH2CH3 |
| C17 group | −CH2CH2COO−Phytyl | −CH2CH2COO−Phytyl | −CH=CHCOOH | −CH=CHCOOH | −CH2CH2COO−Phytyl | −CH2CH2COO−Phytyl |
| C17−C18 bond | Single (chlorin) |
Single (chlorin) |
Double (porphyrin) |
Double (porphyrin) |
Single (chlorin) |
Single (chlorin) |
| Occurrence | Universal | Mostly plants | Various algae | Various algae | Cyanobacteria | Cyanobacteria |
- Structures of chlorophylls
-
chlorophyll a
-
chlorophyll b
-
chlorophyll c1
-
chlorophyll c2
-
chlorophyll d
-
chlorophyll f
Chlorophyll e is reserved for a pigment that has been extracted from algae in 1966 but not chemically described. Besides the lettered chlorophylls, a wide variety of sidechain modifications to the chlorophyll structures are known in the wild. For example, Prochlorococcus, a cyanobacterium, uses 8-vinyl Chl a and b.[19]
Measurement of chlorophyll content
[edit]
Chlorophylls can be extracted from the protein into organic solvents.[20][21][22] In this way, the concentration of chlorophyll within a leaf can be estimated.[23] Methods also exist to separate chlorophyll a and chlorophyll b.
In diethyl ether, chlorophyll a has approximate absorbance maxima of 430 nm and 662 nm, while chlorophyll b has approximate maxima of 453 nm and 642 nm.[24] The absorption peaks of chlorophyll a are at 465 nm and 665 nm. Chlorophyll a fluoresces at 673 nm (maximum) and 726 nm. The peak molar absorption coefficient of chlorophyll a exceeds 105 M−1 cm−1, which is among the highest for small-molecule organic compounds.[25] In 90% acetone-water, the peak absorption wavelengths of chlorophyll a are 430 nm and 664 nm; peaks for chlorophyll b are 460 nm and 647 nm; peaks for chlorophyll c1 are 442 nm and 630 nm; peaks for chlorophyll c2 are 444 nm and 630 nm; peaks for chlorophyll d are 401 nm, 455 nm and 696 nm.[26]
Ratio fluorescence emission can be used to measure chlorophyll content. By exciting chlorophyll a fluorescence at a lower wavelength, the ratio of chlorophyll fluorescence emission at 705±10 nm and 735±10 nm can provide a linear relationship of chlorophyll content when compared with chemical testing. The ratio F735/F700 provided a correlation value of r2 0.96 compared with chemical testing in the range from 41 mg m−2 up to 675 mg m−2. Gitelson also developed a formula for direct readout of chlorophyll content in mg m−2. The formula provided a reliable method of measuring chlorophyll content from 41 mg m−2 up to 675 mg m−2 with a correlation r2 value of 0.95.[27]
The Dualex is an optical sensor used in plant science and agriculture for the assessment of chlorophyll contents in leaves. This device allows researchers to perform real-time and non-destructive measurements.[28]
Biosynthesis
[edit]In some plants, chlorophyll is derived from glutamate and is synthesised along a branched biosynthetic pathway that is shared with heme and siroheme.[29][30][31] Chlorophyll synthase[32] is the enzyme that completes the biosynthesis of chlorophyll a:[33][34]
- chlorophyllide a + phytyl diphosphate chlorophyll a + diphosphate
This conversion forms an ester of the carboxylic acid group in chlorophyllide a with the 20-carbon diterpene alcohol phytol. Chlorophyll b is made by the same enzyme acting on chlorophyllide b. The same is known for chlorophyll d and f, both made from corresponding chlorophyllides ultimately made from chlorophyllide a.[35]
In Angiosperm plants, the later steps in the biosynthetic pathway are light-dependent. Such plants are pale (etiolated) if grown in darkness. Non-vascular plants and green algae have an additional light-independent enzyme and grow green even in darkness.[36]
Chlorophyll is bound to proteins. Protochlorophyllide, one of the biosynthetic intermediates, occurs mostly in the free form and, under light conditions, acts as a photosensitizer, forming free radicals, which can be toxic to the plant. Hence, plants regulate the amount of this chlorophyll precursor. In angiosperms, this regulation is achieved at the step of aminolevulinic acid (ALA), one of the intermediate compounds in the biosynthesis pathway. Plants that are fed by ALA accumulate high and toxic levels of protochlorophyllide; so do the mutants with a damaged regulatory system.[37]
Senescence and the chlorophyll cycle
[edit]The process of plant senescence involves the degradation of chlorophyll: for example the enzyme chlorophyllase (EC 3.1.1.14) hydrolyses the phytyl sidechain to reverse the reaction in which chlorophylls are biosynthesised from chlorophyllide a or b. Since chlorophyllide a can be converted to chlorophyllide b and the latter can be re-esterified to chlorophyll b, these processes allow cycling between chlorophylls a and b. Moreover, chlorophyll b can be directly reduced (via 71-hydroxychlorophyll a) back to chlorophyll a, completing the cycle.[38][39] In later stages of senescence, chlorophyllides are converted to a group of colourless tetrapyrroles known as nonfluorescent chlorophyll catabolites (NCC's) with the general structure:
These compounds have also been identified in ripening fruits and they give characteristic autumn colours to deciduous plants.[39][40]
Distribution
[edit]Chlorophyll maps from 2002 to 2024, provided by NASA, show milligrams of chlorophyll per cubic meter of seawater each month.[41] Places where chlorophyll amounts are very low, indicating very low numbers of phytoplankton, are blue. Places where chlorophyll concentrations are high, meaning many phytoplankton were growing, are yellow. The observations come from the Moderate Resolution Imaging Spectroradiometer (MODIS) on NASA's Aqua satellite. Land is dark gray, and places where MODIS could not collect data because of sea ice, polar darkness, or clouds are light gray. The highest chlorophyll concentrations, where tiny surface-dwelling ocean plants are, are in cold polar waters or in places where ocean currents bring cold water to the surface, such as around the equator and along the shores of continents. It is not the cold water itself that stimulates the phytoplankton. Instead, the cool temperatures are often a sign that the water has welled up to the surface from deeper in the ocean, carrying nutrients that have built up over time. In polar waters, nutrients accumulate in surface waters during the dark winter months when plants cannot grow. When sunlight returns in the spring and summer, the plants flourish in high concentrations.[41]
Uses
[edit]Culinary
[edit]Synthetic chlorophyll is registered as a food additive colorant, and its E number is E140. Chefs use chlorophyll to color a variety of foods and beverages green, such as pasta and spirits. Absinthe gains its green color naturally from the chlorophyll introduced through the large variety of herbs used in its production.[42] Chlorophyll is not soluble in water, and it is first mixed with a small quantity of vegetable oil to obtain the desired solution.[citation needed]
In marketing
[edit]In years 1950–1953 in particular, chlorophyll was used as a marketing tool to promote toothpaste, sanitary towels, soap and other products. This was based on claims that it was an odor blocker — a finding from research by F. Howard Westcott in the 1940s — and the commercial value of this attribute in advertising led to many companies creating brands containing the compound. However, it was soon determined that the hype surrounding chlorophyll was not warranted and the underlying research may even have been a hoax. As a result, brands rapidly discontinued its use. In the 2020s, chlorophyll again became the subject of unsubstantiated medical claims, as social media influencers promoted its use in the form of "chlorophyll water", for example.[43]
See also
[edit]- Bacteriochlorophyll, related compounds in phototrophic bacteria
- Chlorophyllin, a semi-synthetic derivative of chlorophyll
- Deep chlorophyll maximum
- Chlorophyll fluorescence, to measure plant stress
- Purple Earth hypothesis, a scientific hypothesis that explains the evolution of red-blue spectral affinity of chlorophyll.
References
[edit]- ^ a b Virtanen O, Constantinidou E, Tyystjärvi E (2020). "Chlorophyll does not reflect green light – how to correct a misconception". Journal of Biological Education. 56 (5): 1–8. doi:10.1080/00219266.2020.1858930.
- ^ May P. "Chlorophyll". University of Bristol.
- ^ "chlorophyll". Online Etymology Dictionary.
- ^ Muneer S, Kim EJ, Park JS, Lee JH (March 2014). "Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.)". International Journal of Molecular Sciences. 15 (3): 4657–70. doi:10.3390/ijms15034657. PMC 3975419. PMID 24642884.
- ^ Speer BR (1997). "Photosynthetic Pigments". UCMP Glossary (online). University of California Museum of Paleontology. Retrieved 2010-07-17.
- ^ See:
- Delépine M [in French] (September 1951). "Joseph Pelletier and Joseph Caventou". Journal of Chemical Education. 28 (9): 454. Bibcode:1951JChEd..28..454D. doi:10.1021/ed028p454.
- Pelletier PJ, Caventou JB (1817). "Notice sur la matière verte des feuilles" [Notice on the green material in leaves]. Journal de Pharmacie (in French). 3: 486–491. On p. 490, the authors propose a new name for chlorophyll. From p. 490: "Nous n'avons aucun droit pour nommer une substance connue depuis long-temps, et à l'histoire de laquelle nous n'avons ajouté que quelques faits ; cependant nous proposerons, sans y mettre aucune importance, le nom de chlorophyle, de chloros, couleur, et φύλλον, feuille : ce nom indiquerait le rôle qu'elle joue dans la nature." (We have no right to name a substance [that has been] known for a long time, and to whose story we have added only a few facts ; however, we will propose, without giving it any importance, the name chlorophyll, from chloros, color, and φύλλον, leaf : this name would indicate the role that it plays in nature.)
- ^ Willstätter R (1906). "Zur Kenntniss der Zusammensetzung des Chlorophylls" [Contribution to the knowledge of the composition of chlorophyll]. Annalen der Chemie (in German). 350 (1–2): 48–82. doi:10.1002/jlac.19063500103.
From p. 49: "Das Hauptproduct der alkalischen Hydrolyse bilden tiefgrüne Alkalisalze. In ihnen liegen complexe Magnesiumverbindungen vor, die das Metall in einer gegen Alkali auch bei hoher Temperatur merkwürdig widerstandsfähigen Bindung enthalten." (Deep green alkali salts form the main product of alkali hydrolysis. In them, complex magnesium compounds are present, which contain the metal in a bond that is extraordinarily resistant to alkali even at high temperature.)
- ^ a b Motilva MJ (2008). "Chlorophylls – from functionality in food to health relevance". 5th Pigments in Food congress- for quality and health (Print). University of Helsinki. ISBN 978-952-10-4846-3.
- ^ Woodward RB, Ayer WA, Beaton JM, Bickelhaupt F, Bonnett R, Buchschacher P, et al. (July 1960). "The total synthesis of chlorophyll" (PDF). Journal of the American Chemical Society. 82 (14): 3800–3802. doi:10.1021/ja01499a093. Archived (PDF) from the original on 2011-04-10.
- ^ Fleming I (14 October 1967). "Absolute Configuration and the Structure of Chlorophyll". Nature. 216 (5111): 151–152. Bibcode:1967Natur.216..151F. doi:10.1038/216151a0. S2CID 4262313.
- ^ Woodward RB, Ayer WA, Beaton JM, Bickelhaupt F, Bonnett R, Buchschacher P, et al. (1990). "The total synthesis of chlorophyll a". Tetrahedron. 46 (22): 7599–7659. doi:10.1016/0040-4020(90)80003-Z.
- ^ Jabr F (August 2010). "A New Form of Chlorophyll?". Scientific American.
- ^ "Infrared chlorophyll could boost solar cells". New Scientist. 19 August 2010. Retrieved 15 April 2012.
- ^ a b Chen M, Schliep M, Willows RD, Cai ZL, Neilan BA, Scheer H (September 2010). "A red-shifted chlorophyll". Science. 329 (5997): 1318–9. Bibcode:2010Sci...329.1318C. doi:10.1126/science.1191127. PMID 20724585. S2CID 206527174.
- ^ Carter JS (1996). "Photosynthesis". University of Cincinnati. Archived from the original on 2013-06-29.
- ^ "Photosynthesis, Chloroplast | Learn Science at Scitable". www.nature.com. Retrieved 2024-10-19.
- ^ Scheer H (2006). "An Overview of Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications". Chlorophylls and Bacteriochlorophylls. Advances in Photosynthesis and Respiration. Vol. 25. pp. 1–26. doi:10.1007/1-4020-4516-6_1. ISBN 978-1-4020-4515-8.
- ^ Taniguchi M, Lindsey JS (January 2017). "Synthetic Chlorins, Possible Surrogates for Chlorophylls, Prepared by Derivatization of Porphyrins". Chemical Reviews. 117 (2): 344–535. doi:10.1021/acs.chemrev.5b00696. OSTI 1534468. PMID 27498781.
- ^ Chen M (2019). "Chlorophylls d and f: Synthesis, occurrence, light-harvesting, and pigment organization in chlorophyll-binding protein complexes". Advances in Botanical Research. 90: 121–139. doi:10.1016/bs.abr.2019.03.006. ISBN 9780081027523. S2CID 149632511.
- ^ Marker AF (1972). "The use of acetone and methanol in the estimation of chlorophyll in the presence of phaeophytin in plant". Freshwater Biology. 2 (4): 361–385. doi:10.1111/j.1365-2427.1972.tb00377.x.
- ^ Jeffrey SW, Shibata (February 1969). "Some Spectral Characteristics of Chlorophyll c from Tridacna crocea Zooxanthellae". Biological Bulletin. 136 (1): 54–62. doi:10.2307/1539668. JSTOR 1539668.
- ^ Gilpin L (21 March 2001). "Methods for analysis of benthic photosynthetic pigment". School of Life Sciences, Napier University. Archived from the original on April 14, 2008. Retrieved 2010-07-17.
- ^ Cate TM, Perkins TD (October 2003). "Chlorophyll content monitoring in sugar maple (Acer saccharum)". Tree Physiology. 23 (15): 1077–9. doi:10.1093/treephys/23.15.1077. PMID 12975132.
- ^ Gross J (1991). Pigments in vegetables: chlorophylls and carotenoids. Van Nostrand Reinhold. ISBN 978-0442006570.
- ^ Porra RJ, Thompson WA, Kriedemann PE (1989). "Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 975 (3): 384–394. doi:10.1016/S0005-2728(89)80347-0.
- ^ Larkum AW, Douglas S, Raven JA, eds. (2003). Photosynthesis in algae. London: Kluwer. ISBN 978-0-7923-6333-0.
- ^ Gitelson AA, Buschmann C, Lichtenthaler HK (1999). "The Chlorophyll Fluorescence Ratio F735/F700 as an Accurate Measure of Chlorophyll Content in Plants". Remote Sens. Environ. 69 (3): 296–302. Bibcode:1999RSEnv..69..296G. doi:10.1016/S0034-4257(99)00023-1.
- ^ Cerovic, Zoran G. (October 2012). "New proximal sensors of vegetation: towards a non destructive quantitative estimation of plant constituents" (PDF). Ebernburg. Archived from the original (PDF) on 2014-08-22. Retrieved 2024-07-12.
- ^ Battersby AR (December 2000). "Tetrapyrroles: the pigments of life". Natural Product Reports. 17 (6): 507–26. doi:10.1039/B002635M. PMID 11152419.
- ^ Akhtar M (2007). "The Modification of Acetate and Propionate Side Chains During the Biosynthesis of Haem and Chlorophylls: Mechanistic and Stereochemical Studies". Ciba Foundation Symposium 180 - the Biosynthesis of the Tetrapyrrole Pigments. Novartis Foundation Symposia. Vol. 180. pp. 131–155. doi:10.1002/9780470514535.ch8. ISBN 9780470514535. PMID 7842850.
- ^ Willows RD (June 2003). "Biosynthesis of chlorophylls from protoporphyrin IX". Natural Product Reports. 20 (3): 327–41. doi:10.1039/B110549N. PMID 12828371.
- ^ Schmid HC, Rassadina V, Oster U, Schoch S, Rüdiger W (November 2002). "Pre-loading of chlorophyll synthase with tetraprenyl diphosphate is an obligatory step in chlorophyll biosynthesis" (PDF). Biological Chemistry. 383 (11): 1769–78. doi:10.1515/BC.2002.198. PMID 12530542. S2CID 3099209.
- ^ Eckhardt U, Grimm B, Hörtensteiner S (September 2004). "Recent advances in chlorophyll biosynthesis and breakdown in higher plants". Plant Molecular Biology. 56 (1): 1–14. doi:10.1007/s11103-004-2331-3. PMID 15604725. S2CID 21174896.
- ^ Bollivar DW (November 2006). "Recent advances in chlorophyll biosynthesis". Photosynthesis Research. 90 (2): 173–94. doi:10.1007/s11120-006-9076-6. PMID 17370354. S2CID 23808539.
- ^ Tsuzuki Y, Tsukatani Y, Yamakawa H, Itoh S, Fujita Y, Yamamoto H (March 2022). "Effects of Light and Oxygen on Chlorophyll d Biosynthesis in a Marine Cyanobacterium Acaryochloris marina". Plants. 11 (7): 915. doi:10.3390/plants11070915. PMC 9003380. PMID 35406896.
- ^ Muraki N, Nomata J, Ebata K, Mizoguchi T, Shiba T, Tamiaki H, Kurisu G, Fujita Y (May 2010). "X-ray crystal structure of the light-independent protochlorophyllide reductase". Nature. 465 (7294): 110–4. Bibcode:2010Natur.465..110M. doi:10.1038/nature08950. PMID 20400946. S2CID 4427639.
- ^ Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K (October 2001). "FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana". Proceedings of the National Academy of Sciences of the United States of America. 98 (22): 12826–31. Bibcode:2001PNAS...9812826M. doi:10.1073/pnas.221252798. JSTOR 3056990. PMC 60138. PMID 11606728.
- ^ "Chlorophyll Cycle". IUBMB. 2011. Retrieved 2020-06-04.
- ^ a b Hörtensteiner S (2006). "Chlorophyll degradation during senescence". Annual Review of Plant Biology. 57: 55–77. doi:10.1146/annurev.arplant.57.032905.105212. PMID 16669755.
- ^ Müller T, Ulrich M, Ongania KH, Kräutler B (2007). "Colorless tetrapyrrolic chlorophyll catabolites found in ripening fruit are effective antioxidants". Angewandte Chemie. 46 (45): 8699–702. doi:10.1002/anie.200703587. PMC 2912502. PMID 17943948.
- ^ a b "Chlorophyll : Global Maps". Earthobservatory.nasa.gov. Retrieved 5 November 2024.
- ^ Adams J (2004). Hideous absinthe : a history of the devil in a bottle. United Kingdom: I.B.Tauris, 2004. p. 22. ISBN 978-1860649202.
- ^ O'Hagan, Lauren Alex (2022). "All that glistens is not (Green) gold: Historicising the contemporary chlorophyll fad through a multimodal analysis of Swedish marketing, 1950–1953". Journal of Historical Research in Marketing. 14 (3): 374–398. doi:10.1108/jhrm-11-2021-0057.