Calcium iodate
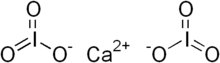
| |
| Names | |
|---|---|
| IUPAC name
Calcium diiodate
| |
| Other names
Lautarite
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.265 |
| EC Number |
|
| E number | E916 (glazing agents, ...) |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Ca(IO3)2 | |
| Molar mass | 389.88 g/mol (anhydrous) 407.90 g/mol (monohydrate) |
| Appearance | white solid |
| Density | 4.519 g/cm3 (monohydrate) |
| Melting point | 540 °C (1,004 °F; 813 K) (monohydrate) |
| Boiling point | decomposes |
| 0.09 g/100 mL (0 °C) 0.24 g/100 mL (20 °C) 0.67 g/100 mL (90 °C) | |
Solubility product (Ksp)
|
7.1×10−7 |
| Solubility | soluble in nitric acid insoluble in alcohol |
| Structure | |
| monoclinic (anhydrous) cubic (monohydrate) orthorhombic (hexahydrate) | |
| Hazards | |
| Flash point | non-flammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Calcium iodates are inorganic compound composed of calcium and iodate anion. Two forms are known, anhydrous Ca(IO3)2 and the monohydrate Ca(IO3)2(H2O). Both are colourless salts that occur naturally as the minerals called lautarite and bruggenite, respectively. A third mineral form of calcium iodate is dietzeite, a salt containing chromate with the formula Ca2(IO3)2CrO4.[1]
Production and reactions
Lautarite, described as the most important mineral source of iodine, is mined in the Atacama Desert.[1] Processing of the ore entails reduction of its aqueous extracts with sodium bisulfite to give sodium iodide. Via a comproportionation reaction, the sodium iodide is combined with the iodate salt to produce elemental iodine.[1] Calcium iodate can be produced by the anodic oxidation of calcium iodide or by passing chlorine into a hot solution of lime in which iodine has been dissolved.
Uses
Calcium iodate can also be used as an iodine supplement in chicken feed.[1]
Calcium iodate is used in the manufacture of disinfectants, antiseptics, and deodorants.[2][3]
References
- ^ a b c d Lyday, Phyllis A.; Tatsuo Kaiho"Iodine and Iodine Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2015, Wiley-VCH, Weinheim, doi:10.1002/14356007.a14_381.pub2 Vol. A14 pp. 382–390.
- ^ "Calcium Iodate". chemicalland21.com.
- ^ Calcium iodate[dead link] from the Online Medical Dictionary
