Cyclopropane
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Cyclopropane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.771 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6 | |||
| Molar mass | 42.08 g/mol | ||
| Density | 1.879 g/L (1 atm, 0 °C) | ||
| Melting point | −128 °C (−198 °F; 145 K) | ||
| Boiling point | −33 °C (−27 °F; 240 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Highly flammable Asphyxiant | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms. Cyclopropane and propene have the same empirical formula but have different structures, making them structural isomers.
The bonds between the carbon atoms are considerably weaker than in a typical carbon-carbon bond, yielding reactivity similar to or greater than alkenes. Baeyer strain theory explains why: the angle strain from the 60° angle between the carbon atoms (less than the normal angle of 109.5° for bonds between atoms with sp3 hybridised orbitals) reduces the compound's carbon-carbon bond energy, making it more reactive than other cycloalkanes such as cyclohexane and cyclopentane. The molecule also has torsional strain due to the eclipsed conformation of its hydrogen atoms. It is somewhat stabilized by some pi character in its carbon-carbon bonds, indicated by the Walsh orbital description whereas it is modeled as a three-center-bonded orbital combination of methylene carbenes [citation needed]. Bent bonds also describe the bonding in cyclopropane.
The smallest polycyclic compounds contain multiple fused cyclopropane rings. Tetrahedrane contains four fused cyclopropane rings which form the faces of a tetrahedron. [1.1.1]Propellane contains three cyclopropane rings which share a single central carbon-carbon bond.
Cyclopropane is an anaesthetic when inhaled. In modern anaesthetic practice, it has been superseded by other agents, due to its extreme reactivity under normal conditions: when the gas is mixed with oxygen there is a significant risk of explosion.
History
Cyclopropane was discovered in 1881 by August Freund, who also proposed the right structure for the new substance in his first paper. Freund reacted 1,3-dibromopropane with sodium, the reaction is an intramolecular Wurtz reaction leading directly to cyclopropane.[2][3] The yield of the reaction can be improved by the use of zinc instead of sodium.[4] Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929;[5] industrial production had begun by 1936.[6]
Safety
Because of the strain in the carbon-carbon bonds of cyclopropane, the molecule has an enormous amount of potential energy. In pure form, it will break down to form linear hydrocarbons, including "normal", non-cyclic propene. This decomposition is potentially explosive, especially if the cyclopropane is liquified, pressurized, or contained within tanks. Explosions of cyclopropane and oxygen are even more powerful, because the energy released by the formation of propene is compounded by the energy released via the oxidation of the carbon and hydrogen present.
At room temperature, sufficient volumes of liquified cyclopropane will self-detonate. To guard against this, the liquid is shipped in cylinders filled with tungsten wool, which prevents high-speed collisions between molecules and vastly improves stability. Pipes to carry cyclopropane must likewise be of small diameter, or else filled with unreactive metal or glass wool, to prevent explosions. Even if these precautions are followed, cyclopropane is dangerous to handle and manufacture, and is no longer used for anaesthesia.
Cyclopropanes
Cyclopropanes are a class of organic compounds sharing the common cyclopropane ring, in which one or more hydrogens may be substituted. These compounds are found in biomolecules; for instance, the pyrethrum insecticides (found in certain Chrysanthemum species) contain a cyclopropane ring.
Organic synthesis
Cyclopropanes can be prepared in the laboratory by organic synthesis in various ways and many methods are simply called cyclopropanation.
By coupling
Cyclopropanes can be obtained by an intramolecular coupling reaction in the addition of sodium to 1,3-dibromopropane in the Freund reaction (1881)[2][3][6] or zinc to 1,3-dichloropropane in the Gustavson reaction(1887)[4][7] or by intramolecular Wurtz coupling for example in the synthesis of bicyclo[1.1.0]butane[8]
From alkenes
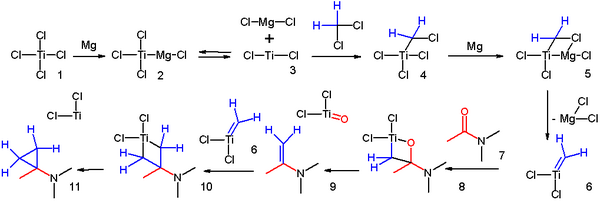
The addition to an alkene of a zinc carbenoid takes place in the Simmons-Smith reaction (1958) for example to cinnamyl alcohol.[9] In one adaptation[10] an amide is reacted with two equivalents of dichloromethane aided by titanium tetrachloride and magnesium:

- a possible reaction mechanism for this cyclopropanation was proposed[11]:
The carbene can also be a regular carbene such as dibromocarbene in the synthesis of propellane[12] or carbene precursor methyl diazoacetate[13]
The addition of a carbene to an alkene is a form of cheletropic reaction.
By nucleophilic displacement
In nucleophilic displacement a leaving group is displaced by a carbon nucleophile in a 1,3 relationship, for example the synthesis of cyclopropylacetylene from 5-chloro-1-pentyne.[14] Another example can be found in the Bingel reaction. An asymmetric reaction creating three stereocenters is demonstrated in a reaction of cyclohexenone with bromonitromethane assisted by trans-2,5-dimethylpiperazine as a base and a pyrrolidine based tetrazole organocatalyst[15][16]:
Rearrangements
Rearrangement reaction of certain cyclobutane compounds can result in cyclopropanes for instance the conversion of 1,2-cyclobutanediol to cyclopropanecarboxaldehyde[17], the di-pi-methane rearrangement is a photochemical rearrangement reaction of 1,4-dienes to vinylcyclopropanes [18]
Other
In the Kulinkovich reaction esters react with Grignards in presence of a titanium alkoxide. Extrusion of nitrogen takes place in certain pyrazolines in the so called Kishner cyclopropane synthesis [19][20]
Organic reactions
Although cyclopropanes are formally cycloalkanes, they are very reactive due to considerable strain energy and due to double bond character.
Electrophilic addition
Cyclopropanes react with mineral acids such as HX in electrophilic addition (very much like a double bond) to linear alkyl halides with ring-opening. Substituted cyclopropanes follow Markovnikov's rule.[21]
Cycloadditions
Cyclopropyl groups participate in cycloaddition reactions such as the formal [5+2]cycloaddition shown below:
This asymmetric synthesis is catalyzed by a rhodium BINAP system with 96% enantiomeric excess[22]. Also see the vinylcyclopropane rearrangement.
Rearrangements
Cyclopropyl groups also engage in many rearrangement reactions. An extreme example is found in the compound bullvalene. A cyclopropane ring is an intermediate in the Favorskii rearrangement. Certain methylenecyclopropanes are found to convert to cyclobutenes[23]:
This reaction is catalyzed by platinum(II) chloride in a carbon monoxide environment. The proposed reaction mechanism is supported by deuterium labeling[24].
In another version of the same reaction[25] the catalyst is PdBr2 is prepared in situ from palladium(II) acetate and copper(II) bromide and the solvent is toluene.
Molecular orbitals
Bonding between the carbon centers in cyclopropane is generally described by invoking bent bonds.[26]
References
- ^ Merck Index, 11th Edition, 2755.
- ^ a b August Freund (1881). "Über Trimethylen". Journal für Praktische Chemie. 26 (1): 625–635. doi:10.1002/prac.18820260125.
- ^ a b August Freund (1882). "Über Trimethylen". Monatshefte für Chemie. 3 (1): 625–635. doi:10.1007/BF01516828.
- ^ a b G. Gustavson (1887). "Ueber eine neue Darstellungsmethode des Trimethylens". J. Prakt. Chem. 36: 300–305. doi:10.1002/prac.18870360127.
- ^ G. H. W. Lucas and V. E. Henderson (1 August 1929). "A New Anæsthetic: Cyclopropane". Can Med Assoc J. 21 (2): 173–5. PMC 1710967.
- ^ a b H. B. Hass, E. T. McBee, and G. E. Hinds (1936). "Synthesis of Cyclopropane". Industrial & Engineering Chemistry. 28 (10): 1178–81. doi:10.1021/ie50322a013.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Freund Reaction
- ^ Bicyclo[1.1.0]butane Gary M. Lampman and James C. Aumiller Organic Syntheses, Coll. Vol. 6, p.133 (1988); Vol. 51, p.55 (1971) Link.
- ^ Cyclopropanemethanol, 2-phenyl-, (1S-trans)- André B. Charette and Hélène Lebel Organic Syntheses, Coll. Vol. 10, p.613 (2004); Vol. 76, p.86 (1999)Link
- ^ Unusual Ambiphilic Carbenoid Equivalent in Amide Cyclopropanation Kuo-Wei Lin, Shiuan Yan, I-Lin Hsieh, and Tu-Hsin Yan Org. Lett.; 2006; 8(11) pp 2265 - 2267; Abstract
- ^ Reaction mechanism: Magnesium reacts with titanium tetrachloride 1 to Grignard reagent 2 in equilibrium with divalent Titanium(II) chloride 3 which adds to dichloromethane to adduct 4. Another insertion of magnesium and loss of magnesium dichloride gives the Schrock carbene 6 which reacts with the carbonyl group in amide 7. Loss of titanium oxychloride gives the enamine 9 which continuous to react with another carbene and finally to the cyclopropane. Notes: Instead of chloride, titanium can also be coordinated to solvent. In equally plausible mechanisms the intermediates are Simmons-Smith like
- ^ [1.1.1]propellane Kathleen R. Mondanaro and William P. Dailey Organic Syntheses, Coll. Vol. 10, p.658 (2004); Vol. 75, p.98 (1998) Link
- ^ Butanoic acid, 3,3-dimethyl-4-oxo-, methyl ester Hans-Ulrich Reissig, Ingrid Reichelt, and Thomas Kunz Organic Syntheses, Coll. Vol. 9, p.573 (1998); Vol. 71, p.189 (1993). Link
- ^ Cyclopropylaetylene Edward G. Corley, Andrew S. Thompson, Martha Huntington Organic Syntheses, Coll. Vol. 10, p.456 (2004); Vol. 77, p.231 (2000) Link.
- ^ A new asymmetric organocatalytic nitrocyclopropanation reaction Henriette M. Hansen, Deborah A. Longbottom and Steven V. Ley Chem. Commun., 2006, 4838 - 4840, doi:10.1039/b612436b
- ^ The initial reaction is a Michael addition. Solvent dichloromethane, 64% enantiomeric excess
- ^ J. P. Barnier, J. Champion, and J. M. Conia Organic Syntheses, Coll. Vol. 7, p.129 (1990); Vol. 60, p.25 (1981) Link.
- ^ IUPAC Gold book definition
- ^ N. M. Kishner, A. Zavadovskii, J. Russ. Phys. Chem. Soc. 43, 1132 (1911).
- ^ For an example see: phenylcyclopropane in Organic Syntheses, Coll. Vol. 5, p.929 (1973); Vol. 47, p.98 (1967). http://orgsynth.org/orgsyn/pdfs/CV5P0929.pdf
- ^ Advanced organic Chemistry, Reactions, mechanisms and structure 3ed. Jerry March ISBN 0-471-85472-7
- ^ Asymmetric Catalysis of the [5 + 2] Cycloaddition Reaction of Vinylcyclopropanes and -Systems Paul A. Wender, Lars O. Haustedt, Jaehong Lim, Jennifer A. Love, Travis J. Williams, and Joo-Yong Yoon J. Am. Chem. Soc.; 2006; 128(19) pp 6302 – 6303; Abstract
- ^ PtCl2-Catalyzed Rearrangement of Methylenecyclopropanes Alois Fürstner and Christophe Aïssa J. Am. Chem. Soc.; 2006; 128(19) pp 6306 -6307; Abstract
- ^ Reaction mechanism: The starting compound contains one deuterium atom (D), in the first step PtCl2 coordinates to the double bond in 1a. The next step is oxidative addition to 1b which is a non-classical ion. This intermediate rearranges to the cyclobutane carbocation 1c which has also some carbene character through one of its resonance structures. The next step is a deuterium migration to the more stable benzylic carbocation after which the cyclobutene is liberated.
- ^ Palladium-Catalyzed Ring Enlargement of Aryl-Substituted Methylenecyclopropanes to Cyclobutenes Min Shi, Le-Ping Liu, and Jie Tang J. Am. Chem. Soc.; 2006; 128(23) pp 7430 - 7431; doi:10.1021/ja061749y
- ^ Eric V. Anslyn and Dennis A. Dougherty. Modern Physical Organic Chemistry. 2006. pages 850-852











