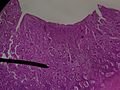
Endometrium
| Endometrium | |
|---|---|
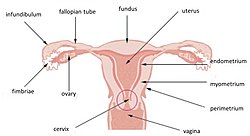 Uterus and uterine tubes. (Endometrium labeled at center right.) | |
| Details | |
| Identifiers | |
| Latin | tunica mucosa uteri |
| MeSH | D004717 |
| TA98 | A09.1.03.027 |
| TA2 | 3521 |
| FMA | 17742 |
| Anatomical terminology | |
The endometrium is the inner epithelial layer, along with its mucous membrane, of the mammalian uterus. It has a basal layer and a functional layer; the functional layer thickens and then is sloughed during the menstrual cycle or estrous cycle. During pregnancy, the glands and blood vessels in the endometrium further increase in size and number. Vascular spaces fuse and become interconnected, forming the placenta, which supplies oxygen and nutrition to the embryo and fetus.[1][2] The presence of commensal bacteria in the uterus and endometrium has been identified.[3]
Structure

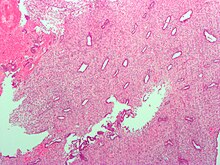
The endometrium consists of a single layer of columnar epithelium plus the stroma on which it rests. The stroma is a layer of connective tissue that varies in thickness according to hormonal influences. Simple tubular uterine glands reach from the endometrial surface through to the base of the stroma, which also carries a rich blood supply of spiral arteries. In a woman of reproductive age, two layers of endometrium can be distinguished. These two layers occur only in endometrium lining the cavity of the uterus, not in the lining of the uterine (Fallopian) tubes:[1][2]
- The functional layer is adjacent to the uterine cavity. This layer is built up after the end of menstruation during the first part of the previous menstrual cycle. Proliferation is induced by estrogen (follicular phase of menstrual cycle), and later changes in this layer are engendered by progesterone from the corpus luteum (luteal phase). It is adapted to provide an optimum environment for the implantation and growth of the embryo. This layer is completely shed during menstruation.
- The basal layer, adjacent to the myometrium and below the functional layer, is not shed at any time during the menstrual cycle, and from it the functional layer develops.
In the absence of progesterone, the arteries supplying blood to the functional layer constrict, so that cells in that layer become ischaemic and die, leading to menstruation.
It is possible to identify the phase of the menstrual cycle by reference to either the ovarian cycle or the uterine cycle by observing histological differences at each phase—for example in the ovarian cycle:
| Phase | Days | Thickness | Epithelium |
|---|---|---|---|
| Menstrual phase | 1–5 | Thin | Absent |
| Follicular phase | 5–14 | Intermediate | Columnar |
| Luteal phase | 15–27 | Thick | Columnar. Also visible are arcuate vessels of uterus |
| Ischemic phase | 27–28 | Columnar. Also visible are arcuate vessels of uterus |
The first studies of flora colonizing the human endometrium were published in 2015, were on special populations, and were too small from which to generalize. In one study of 58 women undergoing hysterectomy, investigators looked for 12 species of bacteria; Lactobacillus and Prevotella were the most commonly detected species and overall density was much lower than vaginal colonization. In another study of 22 infertile women undergoing fertility treatments, Lactobacillus and Flavobacterium were most prevalent, and characteristics of the flora were roughly similar in women who became pregnant and those who didn't.[3]
Function
The endometrium is the innermost glandular layer and functions as a lining for the uterus, preventing adhesions between the opposed walls of the myometrium, thereby maintaining the patency of the uterine cavity. During the menstrual cycle or estrous cycle, the endometrium grows to a thick, blood vessel-rich, glandular tissue layer. This represents an optimal environment for the implantation of a blastocyst upon its arrival in the uterus. The endometrium is central, echogenic (detectable using ultrasound scanners), and has an average thickness of 6.7 mm.
During pregnancy, the glands and blood vessels in the endometrium further increase in size and number. Vascular spaces fuse and become interconnected, forming the placenta, which supplies oxygen and nutrition to the embryo and fetus.
Cycle
The endometrial lining undergoes cyclic regeneration. Humans and the other great apes display the menstrual cycle, whereas most other mammals are subject to an estrous cycle. In both cases, the endometrium initially proliferates under the influence of estrogen. However, once ovulation occurs, in addition to estrogen, the ovary will also start to produce progesterone. This changes the proliferative pattern of the endometrium to a secretory lining. Eventually, the secretory lining provides a hospitable environment for one or more blastocysts.
Upon fertilization, the egg may implant into the uterine wall and provide feedback to the body with human chorionic gonadotropin (HCG). HCG provides continued feedback throughout pregnancy by maintaining the corpus luteum, which will continue its role of releasing progesterone and estrogen. The endometrial lining is either reabsorbed (estrous cycle) or shed (menstrual cycle). In the latter case, the process of shedding involves the breaking down of the lining, the tearing of small connective blood vessels, and the loss of the tissue and blood that had constituted it through the vagina. The entire process occurs over a period of several days. Menstruation may be accompanied by a series of uterine contractions; these help expel the menstrual endometrium.
In case of implantation, however, the endometrial lining is neither absorbed nor shed. Instead, it remains as decidua. The decidua becomes part of the placenta; it provides support and protection for the gestation.
If there is inadequate stimulation of the lining, due to lack of hormones, the endometrium remains thin and inactive. In humans, this will result in amenorrhea, or the absence of a menstrual period. After menopause, the lining is often described as being atrophic. In contrast, endometrium that is chronically exposed to estrogens, but not to progesterone, may become hyperplastic. Long-term use of oral contraceptives with highly potent progestins can also induce endometrial atrophy.[4][5]
In humans, the cycle of building and shedding the endometrial lining lasts an average of 28 days. The endometrium develops at different rates in different mammals. Various factors including the seasons, climate, and stress can affect its development. The endometrium itself produces certain hormones at different stages of the cycle and this affects other parts of the reproductive system.
Clinical significance
Chorionic tissue can result in marked endometrial changes, known as an Arias-Stella reaction, that have an appearance similar to cancer.[6] Historically, this change was diagnosed as endometrial cancer and it is important only in so far as it should not be misdiagnosed as cancer.
- Adenomyosis is the growth of the endometrium into the muscle layer of the uterus (the myometrium).
- Endometriosis is the growth of endometrial tissue outside the uterus.
- Endometrial hyperplasia
- Endometrial cancer is the most common cancer of the human female genital tract.
- Asherman's syndrome, also known as intrauterine adhesions occurs when the basal layer of the endometrium is damaged by instrumentation (e.g., D&C) or infection (e.g., endometrial tuberculosis) resulting in endometrial sclerosis and adhesion formation partially or completely obliterating the uterine cavity.
Thin endometrium may be defined as an endometrial thickness of less than 8 mm. It usually occurs after menopause. Treatments that can improve endometrial thickness include Vitamin E, L-arginine and sildenafil citrate.[7]
Gene expression profiling using cDNA microarray can be used for the diagnosis of endometrial disorders.[8] The European Menopause and Andropause Society (EMAS) released Guidelines with detailed information to assess the endometrium. [9]
Embryo transfer
An endometrial thickness (EMT) of less than 7 mm decreases the pregnancy rate in in vitro fertilization by an odds ratio of approximately 0.4 compared to an EMT of over 7 mm. However, such low thickness rarely occurs, and any routine use of this parameter is regarded as not justified.[10]

Observation of the endometrium by transvaginal ultrasonography is used when administering fertility medication, such as in in vitro fertilization. At the time of embryo transfer, it is favorable to have an endometrium of a thickness of between 7 and 14 mm with a triple-line configuration,[11] which means that the endometrium contains a hyperechoic (usually displayed as light) line in the middle surrounded by two more hypoechoic (darker) lines. A triple-line endometrium reflects the separation of the stratum basalis and functionalis layers, and is also observed in the periovulatory period secondary to rising estradiol levels, and disappears after ovulation.[12]
Additional images
-
The initial stages of human embryogenesis
-
Vertical section of mucous membrane of human uterus.
-
Endometrioid adenocarcinoma from biopsy. H&E stain.
-
Micrograph of the endometrium.
-
Micrograph of decidualized endometrium due to exogenous progesterone. H&E stain.
-
Micrograph of decidualized endometrium due to exogenous progesterone. H&E stain.
-
Micrograph showing endometrial stromal condensation, a finding seen in menses.
See also
- endometrial regenerative cells, also known as endometrial stem cells.
- CYTL1, also known as cytokine-like like protein 1.
References
- ^ a b Blue Histology - Female Reproductive System. School of Anatomy and Human Biology - The University of Western Australia Accessed 20061228 20:35
- ^ a b "Chapter 81 Female Physiology Before Pregnancy and Female Hormones". Textbook of Medical Physiology (11th ed.). Elsevier Saunders. 2006. pp. 1018ff. ISBN 9780721602400.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ a b Franasiak, Jason M.; Scott, Richard T. (2015). "Reproductive tract microbiome in assisted reproductive technologies". Fertility and Sterility. 104 (6): 1364–1371. doi:10.1016/j.fertnstert.2015.10.012. ISSN 0015-0282. PMID 26597628.
- ^ Deligdisch, L. (1993). "Effects of hormone therapy on the endometrium". Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 6 (1): 94–106. PMID 8426860.
- ^ William's Gynecology, McGraw 2008, Chapter 8, Abnormal Uterine Bleeding
- ^ Arias-Stella, J. (Jan 2002). "The Arias-Stella reaction: facts and fancies four decades after". Adv Anat Pathol. 9 (1): 12–23. doi:10.1097/00125480-200201000-00003. PMID 11756756.
- ^ Takasaki A, Tamura H, Miwa I, Taketani T, Shimamura K, Sugino N (April 2010). "Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium". Fertil. Steril. 93 (6): 1851–8. doi:10.1016/j.fertnstert.2008.12.062. PMID 19200982.
- ^ Tseng, L.; Chen, I.; Chen, M.; Yan, H.; Wang, C.; Lee, C. (2010). "Genome-based expression profiling as a single standardized microarray platform for the diagnosis of endometrial disorder: an array of 126-gene model". Fertility and Sterility. 94 (1): 114–119. doi:10.1016/j.fertnstert.2009.01.130. PMID 19328470.
- ^ Dreisler E, Poulsen LG, Antonsen SL, Ceausu I, Depypere H, Erel CT, Lambrinoudaki I, Pérez-López FR, Simoncini T, Tremollieres F, Rees M, Ulrich LG (2013). "EMAS clinical guide: Assessment of the endometrium in peri and postmenopausal women". Maturita. 75 (2): 181–90. doi:10.1016/j.maturitas.2013.03.011. PMID 23619009.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Kasius, A.; Smit, J. G.; Torrance, H. L.; Eijkemans, M. J. C.; Mol, B. W.; Opmeer, B. C.; Broekmans, F. J. M. (2014). "Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis". Human Reproduction Update. 20 (4): 530–541. doi:10.1093/humupd/dmu011. ISSN 1355-4786.
- ^ Zhao, Jing; Zhang, Qiong; Li, Yanping (2012). "The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles". Reproductive Biology and Endocrinology. 10 (1): 100. doi:10.1186/1477-7827-10-100. ISSN 1477-7827.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Baerwald, A. R.; Pierson, R. A. (2004). "Endometrial development in association with ovarian follicular waves during the menstrual cycle". Ultrasound in Obstetrics and Gynecology. 24 (4): 453–460. doi:10.1002/uog.1123. ISSN 0960-7692.
External links
- Anatomy figure: 43:05-15 at Human Anatomy Online, SUNY Downstate Medical Center - "The uterus, uterine tubes and ovary with associated structures."
- Histology image: 18902loa – Histology Learning System at Boston University - "Female Reproductive System uterus, endometrium"
- Swiss embryology (from UL, UB, and UF) gnidation/role02
- Histology image: 20_01 at the University of Oklahoma Health Sciences Center
- Histology at utah.edu. Slide is proliferative phase - click forward to see secretory phase