Infantile hemangioma
| Infantile hemangioma |
|---|
An infantile hemangioma (IH) is one of the most common benign tumors of infancy and occurs in approximately 5–10% of infants.[1][2][3] The word "hemangioma" comes from the Greek haema- (αίμα), "blood"; angeio (αγγείο), "vessel"; -oma (-ωμα), "tumor". Infantile hemangiomas are benign vascular tumors composed of an increased number of unique endothelial cells that line blood vessels.[4] They occur more frequently in female, premature and low birth weight infants.[5][6] Infantile hemangiomas usually appear within the first weeks of life and grow most rapidly during the first three to six months of life.[7][8][9][10] For most hemangiomas, 80% of infantile hemangioma size is generally reached by 3 months of age.[11] Usually, growth and proliferation is complete and involution commences by twelve months of age, however, involution occurs slowly over many years with a majority of infantile hemangioma regression occurring by five years of age.[12][13] Although infantile hemangiomas spontaneously regress over time, some may leave residual redundant fibrofatty tissue, scar, residual telangiectasia, or pigmentary changes.[1][14]
Terminology
The terminology used to define, describe and categorize vascular tumors and malformations has changed over time. The term hemangioma was originally used to describe any vascular tumor-like structure, whether it was present at or around birth or appeared later in life. In 1982, Mulliken and Glowacki proposed a new classification system for vascular anomalies which has been widely accepted and adopted by the International Society for the Study of Vascular Anomalies (ISSVA).[15] This classification system was recently updated in 2015.[16] The classification of vascular anomalies is now based upon cellular features, natural history, and clinical behavior of the lesion. Vascular anomalies are divided into vascular tumors/neoplasms which include infantile hemangiomas, and vascular malformations which include entities with enlarged or abnormal vessels such as capillary malformations (port wine stains), venous malformations, and lymphatic malformations.[16] In 2000, GLUT-1, a specific immunohistochemical marker, was found to be positive in infantile hemangiomas and negative in other vascular tumors or malformations.[4][17][18] This marker has revolutionized the ability to distinguish between infantile hemangioma and other vascular anomalies.[4][19]
Signs and symptoms
Infantile hemangiomas typically develop in the first few weeks or months of life.[1] They are more common in Caucasians, in premature children whose birth weight is less than 3 pounds, in females and in twin births.[5] Early lesions may resemble a red scratch or patch, a white patch, or a bruise. The majority of hemangiomas occur on the head and neck, but they can occur almost anywhere. The appearance and color of the hemangioma depends on its location and depth within the level of the skin.[1]
Superficial hemangiomas are situated higher in the skin and have a bright red, erythematous to reddish-purple appearance. Superficial lesions can be flat and telangiectatic, composed of a macule or patch of small, varied branching capillary blood vessels. They can also be raised and elevated from the skin, forming papules and confluent bright red plaques like raised islands. Infantile hemangiomas have historically been referred to “strawberry hemangiomas” in the past, as raised superficial hemangiomas can look like the side of a strawberry without seeds. Superficial hemangiomas in certain locations, such as the posterior scalp, neck folds and groin/perianal areas are at potential risk of ulceration. Ulcerated hemangiomas can present as black crusted papules or plaques, or painful erosions or ulcers. Ulcerations are prone to secondary bacterial infections which can present with yellow crusting, drainage, pain or odor. Ulcerations are also at risk for bleeding, particularly deep lesions or in areas of friction. Multiple superficial hemangiomas, more than 5 can be associated with extracutaneous hemangiomas, the most common being a liver (hepatic) hemangioma and these infants warrant ultrasound examination.[1]
Deep hemangiomas present as poorly defined, bluish macules that can proliferate into papules, nodules or larger tumors. Proliferating lesions are often compressible, but fairly firm. Many deep hemangiomas may have a few superficial capillaries visible evident over the primary deep component or surrounding venous prominence. Deep hemangiomas have a tendency to develop a little later than superficial hemangiomas and may have longer and later proliferative phases as well. Deep hemangiomas rarely ulcerate, but can cause issues depending on their location, size and growth. Deep hemangiomas near sensitive structures can cause compression of softer surrounding structures during the proliferative phase, such as the external ear canal and the eyelid.[1] Mixed hemangiomas are simply a combination of superficial and deep hemangiomas, and may not be evident for several months. Patients may have any combination of superficial, deep or mixed infantile hemangiomas.
Infantile hemangiomas are often classified as focal/localized, segmental or indeterminate. Focal infantile hemangiomas appear localized to a specific location and appear to arise from a solitary spot. Segmental hemangiomas are larger, appear to encompass a region of the body. Larger or segmental hemangiomas that span over a large area can sometimes have underlying anomalies that may require investigation especially when located on the face, sacrum or pelvis.
Unless there is ulceration, hemangiomas do not tend to bleed and are not painful. Discomfort may arise if the hemangioma is bulky and blocks a vital orifice.[1][5][20][21][22]
-
Hemangioma on forehead showing signs of early regression
-
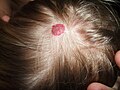
Hemangioma on the scalp of a two-year-old child, in the "rest stage"
-
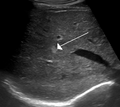
Hemangioma of the liver as seen on ultrasound
-
A liver hemangioma as seen on CT
Causes
The cause of hemangioma is currently unknown; however, several studies have suggested the importance of estrogen signaling in hemangioma proliferation. In 2007, a paper from the Stanford Children's Surgical Laboratory revealed that localized soft tissue hypoxia coupled with increased circulating estrogen after birth may be the stimulus.[23] There is also a hypothesis presented by researchers that maternal placenta embolizes to the fetal dermis during gestation resulting in hemangiomagenesis.[24][25] However, another group of researchers conducted genetic analyses of single-nucleotide polymorphism in hemangioma tissue compared to the mother's DNA that contradicted this hypothesis.[26] Other studies have revealed the role of increased angiogenesis and vasculogenesis in the etiology of hemangiomas.[27] More research is required in order to fully understand the disproportionate nature of hemangioma growth, which will hopefully yield targeted therapeutics to treat its most complicated presentations.
Diagnosis

The majority of infantile hemangiomas (IH) can be diagnosed by history and physical exam.[6] In rare cases, imaging (ultrasound (US) with Doppler, magnetic resonance imaging (MRI)) and/or cytology or histopathology are needed to confirm the diagnosis.[28][18] IH are usually absent at birth or there may be a small area of pallor, telangiectasias, or duskiness. A fully formed mass at birth usually indicates a diagnosis other than IH. Superficial hemangiomas in the upper dermis have a bright red strawberry color, whereas those in the deep dermis and subcutis, deep hemangiomas, may appear blue and be firm or rubbery on palpation. Mixed hemangiomas can have both features.[6] A minimally proliferative IH is an uncommon type of IH that presents with fine macular telangiectasias with an occasional bright-red, papular, proliferative component. Minimally proliferative IH are more common in the lower body.[29]
A precise history of the growth characteristics of the IH can be very helpful in making the diagnosis. In the first 4 to 8 weeks of life IH grow rapidly with primarily volumetric rather than radial growth. This is usually followed by a period of slower growth that can last up to 6–9 months, with 80% of the growth completed by 3 months. Finally, IH involute over a period of years.[11] There are exceptions to these growth characteristics: minimally proliferative IH which do not substantially proliferate[29] and large deep IH in which noticeable growth starts later and lasts longer.[11]
If the diagnosis is not clear based on physical exam and growth history (most often in deep hemangiomas with little cutaneous involvement), then either imaging or histopathology can help confirm the diagnosis.[28][4] On Doppler ultrasound an IH in the proliferative phase appears as a high flow soft-tissue mass usually without direct arteriovenous shunting. On MRI, IH show a well-circumscribed lesion with intermediate and increased signal intensity on T1 and T2-weighted sequences, respectively, and strong enhancement after gadolinium injections. There are fast flow vessels.[28] Tissue for diagnosis can be obtained via fine needle aspiration, skin biopsy, or excisional biopsy.[30] Under the microscope, hemangiomas are unencapsulated aggregates of closely packed, thin-walled capillaries, usually with endothelial lining. Blood-filled vessels are separated by scant connective tissue. Their lumens may be thrombosed and organized. Hemosiderin pigment deposition due to vessel rupture may be observed.[31] The GLUT-1 histochemical marker can be helpful in distinguishing IH from other items on the differential diagnosis, such as vascular malformations.[18]
Complications
The vast majority of hemangiomas are not associated with complications. Hemangiomas may break down on the surface, called ulceration. Ulceration can be painful and problematic. If the ulceration is deep, significant bleeding and infection may occur in rare occasions. If a hemangioma develops in the larynx, breathing can be compromised. If located near the eye, a growing hemangioma may cause an occlusion or deviation of the eye that can lead to amblyopia.[32] Very rarely, extremely large hemangiomas can cause high-output heart failure due to the amount of blood that must be pumped to excess blood vessels. Lesions adjacent to bone may cause erosion of the bone.[1]
The most frequent complaints about hemangiomas stem from psychosocial complications. The condition can affect a person's appearance and provoke attention and malicious reactions from others. Particular problems occur if the lip or nose is involved, as distortions can be difficult to treat surgically. The potential for psychological injury develops from school age onward. It is therefore important to consider treatment before school begins if adequate spontaneous improvement has not occurred. Large hemangiomas can leave visible skin changes secondary to severe stretching that results in altered surface texture.
Large segmental hemangiomas of the head and neck can be associated with a disorder called PHACES syndrome.[33][34] Large segmental hemangiomas over the lumbar spine can be associated with dysraphism, renal, and urogenital problems in association with a disorder called LUMBAR syndrome. Multiple cutaneous hemangiomas in infants may be an indicator for liver hemangiomas. Screening for liver involvement is often recommended in infants with 5 or more skin hemangiomas.[35]
Management of Infantile Hemangioma
Most hemangiomas disappear without treatment, leaving minimal to no visible marks. This may take many years, however, and a significant proportion of lesions may require some form of therapy.[36] Indications for treatment of a hemangioma include functional impairment (ie visual or feeding compromise), bleeding, potentially life-threatening complications (ie airway, cardiac or hepatic disease), and risk of long-term or permanent disfigurement.[37] Large hemangiomas can leave visible skin changes secondary to significant stretching of the skin or alteration of surface texture. When hemangiomas interfere with vision, breathing, or threaten significant disfigurement (most notably facial lesions and, in particular, nose and lips), they are usually treated. Medical therapies are most effective when utilized during the period of most significant hemangioma growth, which corresponds to the first five months of life.[11] Ulcerated hemangiomas, a subset of lesions requiring therapy, are usually treated by addressing wound care, pain and hemangioma growth.[38]
Treatment options for hemangiomas include medical therapies (systemic,intralesional and topical), surgery, and laser therapy. Prior to 2008, the mainstay of therapy for problematic hemangiomas was oral corticosteroids, which are effective and remain an option for patients in whom beta-blocker therapy is contraindicated or poorly tolerated.[39][40][41] Following the serendipitous observation that propranolol, a non-selective beta blocker, is well tolerated and effective for treatment of hemangiomas,[42][43] the agent was studied in a large, randomized controlled trial[44] and was approved by the U.S. Food and Drug Administration for this indication in 2014.[45] Propranolol has subsequently become the first-line systemic medical therapy for treatment of these lesions.[37]
Other systemic therapies which may be effective for hemangioma treatment include vincristine, interferon- and other agents with antiangiogenic properties. Vincristine, which requires central venous access for administration, is traditionally used as a chemotherapy agent, but has been demonstrated to have efficacy against hemangiomas and other childhood vascular tumors, such as Kaposiform hemangioendothelioma and tufted angioma.[46][47] Interferon-alpha 2a and 2b, given via subcutaneous injection, has shown efficacy against hemangiomas,[48] but may result in spastic diplegia in up to 20% of treated children.[49][50] These agents are rarely utilized now in the era of beta blocker therapy for hemangiomas.
Intralesional corticosteroid (usually triamcinolone) injection has been used for small, localized hemangiomas, where it has been demonstrated relatively safe and effective.[51][52] Injection of upper eyelid hemangiomas is controversial, given the reported risk of retinal embolization, possibly related to high injection pressures.[53][54] Topical timolol maleate, a non-selective beta blocker available in a gel-forming solution approved for the treatment of glaucoma, has been increasingly recognized as a safe and effective off-label alternative for treatment of small hemangiomas.[55][56][57] It is generally applied two to three times daily.[58]
Surgical excision of hemangiomas is rarely indicated, and limited to lesions which fail medical therapy (or when it is contraindicated), which are anatomically distributed in a location which is amenable to resection, and in which resection would likely be necessary and the scar will be similar regardless of timing of the surgery.[37][59] Surgery may also be useful for removal of residual fibrofatty tissue (following hemangioma involution) and reconstruction of damaged structures.
Laser therapy, most often the pulsed dye laser (PDL), plays a limited role in hemangioma management.[60] It is most often used for treatment of ulcerated hemangiomas, often in conjunction with topical therapies and wound care, and may speed healing and diminish pain.[61][62] Laser therapy may also be useful for early superficial hemangiomas (although rapidly proliferating lesions may be more prone to ulceration following PDL treatment), and for the treatment of cutaneous telangiectasias which persist following hemangioma involution.[63][64]
Prognosis
In the involution phase, a hemangioma finally begins to diminish in size. While it was previously thought that infantile hemangiomas improved by about 10% each year, newer evidence suggests that maximal improvement and involution is typically reached by 3.5 years of age.[65][12] The majority of hemangiomas resolve by age 10, however, in some patients, the hemangioma doesn't completely resolve. Residual redness may be noted and can be improved with laser therapy, most commonly pulsed dye laser.[66] Ablative fractional resurfacing may be considered for textural skin changes.[67] Hemangiomas, especially those that have gotten very large during the growth phase, may leave behind stretched skin or fibrofatty tissue that may be disfiguring or require future surgical correction. Areas of prior ulceration may leave behind permanent scarring.
Additional long term sequelae stem from the identification of extracutaneous manifestations in association with the infantile hemangioma. For example, a patient with a large facial hemangioma who is found to meet criteria for PHACE syndrome, will require potentially ongoing neurologic, cardiac, and/or ophthalmologic monitoring. In cases of infantile hemangiomas that compromise of vital structures, symptoms may improve with involution of the hemangioma. For example, respiratory distress would improve with involution of a space occupying hemangioma involving the airway and high output heart failure may lessen with involution of a hepatic hemangioma and ultimately treatment may be tapered or discontinued. In other cases, such as an untreated eyelid hemangioma, resultant amblyopia does not improve with involution of the cutaneous lesion. For these reasons, it is important that infants with infantile hemangiomas be evaluated by an appropriate clinician during the early proliferative phase so that risk monitoring and treatment be individualized and outcomes can be optimized.[11][68]
References
- ^ a b c d e f g h Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in Children. New England Journal of Medicine. 1999;341(3):173-181.
- ^ Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008;25(2):168-173.
- ^ Kanada KN, Merin MR, Munden A, Friedlander SF. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- ^ a b c d North PE, Mihm MC Jr. Histopathological diagnosis of infantile hemangiomas and vascular malformations. Facial Plast Surg Clin North Am. 2001;9(4):505-524.
- ^ a b c Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr. 2007;150(3):291-294.
- ^ a b c Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138(12):1567-1576.
- ^ Bivings L. Spontaneous regression of angiomas in children: twenty-two years’ observation covering 236 cases. J Pediatr. 1954;45(6):643-647.
- ^ Bowers RE, Graham EA, Tominson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82(5):667-670.
- ^ Jacobs AH. Strawberry hemangiomas: the natural history of the untreated lesion. Calif Med. 1957;86(1):8-10.
- ^ Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents' photographs tell us. Pediatrics. 2012;130(2):e314-e320.
- ^ a b c d e Chang LC, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Nopper AJ, Frieden IJ; Hemangioma Investigator Group. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122(2):360-367.
- ^ a b Couto RA, Maclellan RA, Zurakowski D, Greene AK. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130(3):619-624.
- ^ Bauland CG, Lüning TH, Smit JM, Zeebregts CJ, Spauwen PH. Untreated hemangiomas: growth pattern and residual lesions. Plast Reconstr Surg. 2011;127(4):1643-1648.
- ^ Jackson R. The natural history of strawberry naevi. J Cutan Med Surg. 1998;2(3):187-189.
- ^ Mulliken JB, Glowacki J.Classification of pediatric vascular lesions. Plast Reconstr Surg. 1982 Jul;70(1):120-1.
- ^ a b Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, Burrows P, Frieden IJ, Garzon MC, Lopez-Gutierrez JC, Lord DJ, Mitchel S, Powell J, Prendiville J, Vikkula M; Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. ISSVA Board and Scientific Committee. Pediatrics. 2015 Jul;136(1):e203-14. doi: 10.1542/peds.2014-3673. Epub 2015 Jun 8. Review.
- ^ North PE, Waner M, James CA, Mizeracki A, Frieden IJ, Mihm MC Jr. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001 Dec;137(12):1607-20.
- ^ a b c North PE, Waner M, Mizeracki A, Mihm MC Jr. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000 Jan;31(1):11-22.
- ^ Leon-Villapalos J, Wolfe K, Kangesu L. Br J GLUT-1: an extra diagnostic tool to differentiate between haemangiomas and vascular malformations. Plast Surg. 2005 Apr;58(3):348-52.
- ^ http://www.lacolon.com/patient-education/gi-associated-hemangiomas-and-vascular-malformations
- ^ http://www.cincinnatichildrens.org/health/h/hemangioma/
- ^ Oakley, Amanda. "Infantile haemangioma.". DermNet NZ. Retrieved February 11, 2013.
- ^ Kleinman ME, Greives MR, Churgin SS et al. (December 2007). "Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma". Arterioscler. Thromb. Vasc. Biol. 27 (12): 2664–70. doi:10.1161/ATVBAHA.107.150284. PMID 17872454.
- ^ Barnés CM, Huang S, Kaipainen A et al. (December 2005). "Evidence by molecular profiling for a placental origin of infantile hemangioma". Proc. Natl. Acad. Sci. U.S.A. 102 (52): 19097–102. doi:10.1073/pnas.0509579102. PMC 1323205. PMID 16365311.
- ^ North PE, Waner M, Brodsky MC (April 2002). "Are infantile hemangiomas of placental origin?". Ophthalmology 109 (4): 633–4. doi:10.1016/S0161-6420(02)01071-0. PMID 11949625.
- ^ Pittman KM, Losken HW, Kleinman ME et al. (November 2006). "No evidence for maternal-fetal microchimerism in infantile hemangioma: a molecular genetic investigation". J. Invest. Dermatol. 126 (11): 2533–8. doi:10.1038/sj.jid.5700516. PMID 16902414.
- ^ Greenberger Sa, Bischoff. Pathogenesis of Infantile Hemangioma. BrJ Dermatol 2013 Jul;169(1) 12-9.
- ^ a b c Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol. 2010 Jun;40(6):895-905. doi: 10.1007/s00247-010-1621-y. Epub 2010 Apr 30. Review. PubMed PMID 20432007.
- ^ a b Suh KY, Frieden IJ. Infantile hemangiomas with minimal or arrested growth: A retrospective case series. Arch Dermatol. 2010 Sep;146(9):971-6.
- ^ Erhardt CA, Vesoulis Z, Kashkari S. Fine needle aspiration cytology of cellular hemangioma of infancy. A case report. Acta Cytol. 2000 Nov-Dec;44(6):1090-4. PubMed PMID 11127741.
- ^ Kumar Vinay: Robbins and Coltran pathologic basis of disease 8ed.. pp. 520-521 Philadelphia: Saunders Elsevier, 2010. ISBN 978-0-8089-2402-9
- ^ Hunzeker C, Geronemus R (2010). Treatment of Superficial Infantile Hemangiomas of the Eyelid Using the 595-nm Pulsed Dye Laser". Dermatol. Surg. 36 (5): 590–597.
- ^ Oza VS, Wang E, Berenstein A et al. (April 2008). "PHACES association: a neuroradiologic review of 17 patients". AJNR Am J Neuroradiol 29 (4): 807–13.
- ^ Heyer GL, Dowling MM, Licht DJ et al. (February 2008). "The cerebral vasculopathy of PHACES syndrome". Stroke 39 (2): 308–16.
- ^ Horii KA, Drolet BA, Frieden IJ, Baselga E, Chamlin SL, Haggstrom AN, Holland KE, Mancini AJ, McCuaig CC, Metry DW, Morel KD, Newell BD, Nopper AJ, Powell J, Garzon MC; Hemangioma Investigator Group. Prospective study of the frequency of hepatic hemangiomas in infants with multiple cutaneous infantile hemangiomas. Pediatr Dermatol. 2011 May-Jun;28(3):245-53. doi: 10.1111/j.1525-1470.2011.01420.x. Epub 2011 Apr 26.
- ^ Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics 2006;118(3):882-7.
- ^ a b c Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics 2015;136(4):e1060-1104.
- ^ Kim HJ, Colombo M, Frieden IJ. Ulcerated hemangiomas: clinical characteristics and response to therapy. J Am Acad Dermatol 2001;44(6):962-72.
- ^ Bennett ML, Fleischer AB, Chamlin SL, et al. Oral corticosteroid use is effective for cutaneous hemangiomas: an evidence-based evaluation. Arch Dermatol 2001;137(9):1208-13.
- ^ Sadan N, Wolach B. Treatment of hemangiomas of infants with high doses of prednisone. J Pediatr 1996;128(1):141-6
- ^ Greene AK, Couto RA. Oral prednisolone for infantile hemangioma: efficacy and safety using a standardized treatment protocol. Plast Reconstr Surg 2011;128(3):743-52.
- ^ Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358(24):2649-51.
- ^ Hogeling M et al. (August 2011). "A randomized controlled trial of propranolol for infantile hemangiomas". Pediatrics 128 (2): e259–e266.
- ^ Leaute-Labreze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 2015;372(8):735-46.
- ^ United States Food and Drug Administration. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205410s000lbl.pdf; accessed 9/29/15.
- ^ Perez Payarols J, Pardo Masferrer J, Gomez Bellvert C. Treatment of life-threatening infantile hemangiomas with vincristine. N Engl J Med 1995;333(1):69.
- ^ Enjolras O, Breviere GM, Roger G, et al. Vincristine treatment for function- and life-threatening infantile hemangioma. Arch Pediatr 2004;11(2):99-107.
- ^ Wilson MW, Hoehn ME, Haik BG, Rieman M, Reiss U (May 2007). "Low-dose cyclophosphamide and interferon alfa 2a for the treatment of capillary hemangioma of the orbit". Ophthalmology 114 (5): 1007–11.
- ^ Barlow CF, Priebe CJ, Mulliken JB, et al. Spastic diplegia as a complication of interferon alfa-2a treatment of hemangiomas of infancy. J Pediatr 1998;132(3 pt 1):527-30.
- ^ Worle H, Maass E, Kohler B, et al. Interferon alpha-2a therapy in haemangiomas of infancy: spastic diplegia as a severe complication. Eur J Pediatr 1999;158(4):344.
- ^ Chen MT, Yeong EK, Horng SY. Intralesional corticosteroid therapy in proliferating head and neck hemangiomas: a review of 155 cases. J Pediatr Surg 2000;35(3):420-3.
- ^ Chantharatanapiboon W. Intralesional corticosteroid therapy in hemangiomas: clinical outcome in 160 cases. J Med Assoc Thai 2008;91(suppl 3):S90-6.
- ^ Ruttum MS, Abrams GW, Harris GJ, et al. Bilateral retinal embolization associated with intralesional corticosteroid injection for capillary hemangioma of infancy. J Pediatr Ophthalmol Strabismus 1993;30(1):4-7.
- ^ Egbert JE, Schwartz GS, Walsh AW. Diagnosis and treatment of an ophthalmic artery occlusion during an intralesional injection of corticosteroid into an eyelid capillary hemangioma. Am J Ophthalmol 1996;121(6):638-42.
- ^ Moehrle M, Leaute-Labreze C, Schmidt V, et al. Topical timolol for small hemangiomas of infancy. Pediatr Dermatol 2013;30(2):245-9.
- ^ Guo S, Ni N (February 2010). "Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution". Arch. Ophthalmol. 128 (2): 255–6.
- ^ Chakkittakandiyii A, Phillips R, Frieden IJ, et al. Timolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas: a retrospective multicenter cohort study. Pediatr Dermatol 2012;29(3):28-31.
- ^ Pope E, Chakkittakandiyil A (May 2010). "Topical timolol gel for infantile hemangiomas: a pilot study". Arch Dermatol 146 (5): 564–5.
- ^ Greene AK. Management of hemangiomas and other vascular tumors. Clin Plast Surg 2011;38(1):45-63.
- ^ Rizzo C, Brightman L, Chapas AM, et al. Outcomes of childhood hemangiomas treated with the pulsed-dye laser with dynamic cooling: a retrospective chart analysis. Dermatol Surg 2009;35(12):1947-54.
- ^ David LR, Malek MM, Argenta LC. Efficacy of pulse dye laser therapy for the treatment of ulcerated haemangiomas: a review of 78 patients. Br J Plast Surg 2003;56(4):317-27.
- ^ Morelli JG, Tan OT, Weston WL. Treatment of ulcerated hemangiomas with the pulsed tunable dye laser. Am J Dis Child 1991;145(9):1062-4.
- ^ Garden JM, Bakus AD, Paller AS. Treatment of cutaneous hemangiomas by the flashlamp-pumped pulsed dye laser: prospective analysis. J Pediatr 1992;120(4 pt 1):555-60.
- ^ Waner M, Suen JY, Dinehart S, et al. Laser photocoagulation of superficial proliferating hemangiomas. J Dermatol Surg Oncol 1994;20(1):43-6.
- ^ Wahrman JE, Honig PJ. Hemangiomas. Pediatr Rev 1994; 15: 266– 271.
- ^ Stier MF, Glick SA, Hirsch RJ. Laser treatment of pediatric vascular lesions: Port wine stains and hemangiomas. J Am Acad Dermatol. 2008 Feb;58(2):261-85.
- ^ Brightman LA, Brauer JA, Terushkin V, Hunzeker C, Reddy KK, Weiss ET, Karen JK, Hale EK, Anolik R, Bernstein L, Geronemus RG. Ablative fractional resurfacing for involuted hemangioma residuum. Arch Dermatol. 2012 Nov;148(11):1294-8.
- ^ Bruckner AL, Frieden IJ. Hemangiomas of infancy. J Am Acad Dermatol. 2003 Apr;48(4):477-93; quiz 494-6.
External links
- Hemangioma Investigator Group
- Hemangioma - Children's Hospital Los Angeles
- Hemangioma - at Web MD
- Hemangioma - Children's Hospital Boston
- Humpath #1990 (Pathology images) at humpath.com
- MedlinePlus Encyclopedia: Hemangioma
- Hepatic Hemangioma at medicinenet.com
- Haemangiomas of infancy at rch.org.au
- Liver Hemangioma at USUHS - MedPix
- Infantile Hemangiomas - Cincinnati Children's Hospital