Holmium(III) oxide

| |
| Names | |
|---|---|
| Other names
Holmium oxide, Holmia
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.820 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Ho2O3 | |
| Molar mass | 377.859 g/mol |
| Appearance | yellow cubic crystals |
| Density | 8.41 g/cm3 |
| Melting point | 2415 °C |
| Boiling point | 3900 °C |
| insoluble | |
| Solubility in acids[1] | soluble |
| Band gap | 5.3 eV [2] |
Refractive index (nD)
|
1.8 [2] |
| Structure | |
| Cubic, cI80, | |
| Ia-3, No. 206 | |
| Thermochemistry | |
Heat capacity (C)
|
115.0 J•mol-1•K-1[1] |
Std molar
entropy (S⦵298) |
158.2 J•mol-1•K-1 |
Std enthalpy of
formation (ΔfH⦵298) |
-1880.7 kJ•mol-1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Holmium(III) oxide, or holmium oxide is a chemical compound of a rare-earth element holmium and oxygen with the formula Ho2O3. Together with dysprosium(III) oxide (Dy2O3) holmium oxide is one of the most powerfully paramagnetic substances known. The oxide, also called holmia, occurs as a component of the related erbium oxide mineral called erbia. Typically the oxides of the trivalent lanthanides coexist in nature and separation of these components requires specialized methods. Holmium oxide is used in making specialty colored glasses. Glass containing holmium oxide and holmium oxide solutions have a series of sharp optical absorption peaks in the visible spectral range. They are therefore traditionally used as a convenient calibration standard for optical spectrophotometers.
Properties
Appearance
Holmium oxide has some fairly dramatic color changes depending on the lighting conditions. In daylight, it is a tannish yellow color. Under trichromatic light, it is a fiery orange red, almost indistinguishable from the way erbium oxide looks under this same lighting. This is related to the sharp emission bands of the phosphors.[3] Holmium oxide has a wide band gap of 5.3 eV[2] and thus should appear colorless. The yellow color originates from abundant lattice defects (such as oxygen vacancies) and is related to internal transitions at the Ho3+ ions.[3]
Crystalline structure
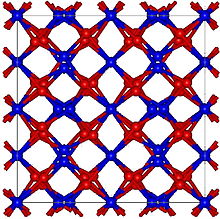
Holmium oxide has a cubic, yet rather complex structure, with many atoms per unit cell and a large lattice constant of 1.06 nm. This structure is characteristic of oxides of heavy rare-earth elements, such as Tb2O3, Dy2O3, Er2O3, Tm2O3, Yb2O3 and Lu2O3. The thermal expansion coefficient of Ho2O3 is also relatively large at 7.4 µm/°C.[4]
Chemical
Treating holmium oxide with hydrogen chloride or with ammonium chloride affords the corresponding holmium chloride:[5]
- Ho2O3 + 6 NH4Cl → 2 HoCl3 + 6 NH3 + 3 H2O
History
Holmium (Holmia, Latin name for Stockholm) was discovered by Marc Delafontaine and Jacques-Louis Soret in 1878 who noticed the aberrant spectrographic absorption bands of the then-unknown element (they called it "Element X").[6][7] Later in 1878, Per Teodor Cleve independently discovered the element while he was working on erbia earth (erbium oxide).[8][9]
Using the method developed by Carl Gustaf Mosander, Cleve first removed all of the known contaminants from erbia. The result of that effort was two new materials, one brown and one green. He named the brown substance holmia (after the Latin name for Cleve's home town, Stockholm) and the green one thulia. Holmia was later found to be the holmium oxide and thulia was thulium oxide.[10]
Occurrence

Holmium oxide occurs in trace amounts in the minerals gadolinite, monazite, and in other rare-earth minerals. Holmium metal readily oxidizes in air; therefore presence of holmium in nature is synonymous with that of holmia. With the abundance of 1.4 mg/kg, holmium is the 56th most abundant element.[10] The main mining areas are China, United States, Brazil, India, Sri Lanka and Australia with reserves of holmium oxide estimated as 400,000 tonnes.[10]
Production
A typical extraction process of holmium oxide can be simplified as follows: The mineral mixtures are crushed and ground. Monazite, because of its magnetic properties can be separated by repeated electromagnetic separation. After separation, it is treated with hot concentrated sulfuric acid to produce water-soluble sulfates of several rare earth elements. The acidic filtrates are partially neutralized with sodium hydroxide to pH 3-4. Thorium precipitates out of solution as hydroxide and is removed. After that, the solution is treated with ammonium oxalate to convert rare earths in to their insoluble oxalates. The oxalates are converted to oxides by annealing. The oxides are dissolved in nitric acid that excludes one of the main components, cerium, whose oxide is insoluble in HNO3.
The most efficient separation routine for holmium oxide from the rare-earths is ion exchange. In this process, rare-earth ions are adsorbed onto suitable ion-exchange resin by exchange with hydrogen, ammonium or cupric ions present in the resin. The rare earth ions are then selectively washed out by suitable complexing agent, such as ammonium citrate or nitrilotracetate.[5]
Applications

Holmium oxide is one of the colorants used for cubic zirconia and glass, providing yellow or red coloring.[11] Glass containing holmium oxide and holmium oxide solutions (usually in perchloric acid) have sharp optical absorption peaks in the spectral range 200-900 nm. They are therefore used as a calibration standard for optical spectrophotometers[12][13] and are available commercially.[14] As most other oxides of rare-earth elements, holmium oxide is used as a specialty catalyst, phosphor and a laser material. Holmium laser operates at wavelength of about 2.08 micrometres, either in pulsed or continuous regime. This laser is eye safe and is used in medicine, LIDARs, wind velocity measurements and atmosphere monitoring.[15]
Health effects
Holmium(III) oxide is, compared to many other compounds, not very dangerous, although repeated overexposure can cause granuloma and hemoglobinemia. It has low oral, dermal and inhalation toxicities and is non-irritating. The acute oral median lethal dose (LD50) is greater than 1 g per kilogram of body weight.[16]
References
- ^ a b Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 4–60. ISBN 0849305942.
- ^ a b c Wiktorczyk, T (2002). "Preparation and optical properties of holmium oxide thin films". Thin Solid Films. 405: 238. doi:10.1016/S0040-6090(01)01760-6.
- ^ a b Su, Yiguo; Li, Guangshe; Chen, Xiaobo; Liu, Junjie; Li, Liping (2008). "Hydrothermal Synthesis of GdVO4:Ho3+ Nanorods with a Novel White-light Emission". Chemistry Letters. 37: 762. doi:10.1246/cl.2008.762.
- ^ Singh, H; Dayal, B (1969). "Precise determination of the lattice parameters of holmium and erbium sesquioxides at elevated temperatures". Journal of the Less Common Metals. 18: 172. doi:10.1016/0022-5088(69)90137-4.
- ^ a b Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. pp. 340, 445. ISBN 0070494398. Retrieved 2009-06-06.
- ^ Jacques-Louis Soret (1878). "Sur les spectres d'absorption ultra-violets des terres de la gadolinite". Comptes rendus de l'Académie des sciences. 87: 1062.
- ^ Jacques-Louis Soret (1879). "Sur le spectre des terres faisant partie du groupe de l'yttria". Comptes rendus de l'Académie des sciences. 89: 521.
- ^ Per Teodor Cleve (1879). "Sur deux nouveaux éléments dans l'erbine". Comptes rendus de l'Académie des sciences. 89: 478.
- ^ Per Teodor Cleve (1879). "Sur l'erbine". Comptes rendus de l'Académie des sciences. 89: 708.
- ^ a b c John Emsley (2001). Nature's building blocks: an A-Z guide to the elements. US: Oxford University Press. pp. 180–181. ISBN 0198503415. Cite error: The named reference "history" was defined multiple times with different content (see the help page).
- ^ "Cubic zirconia". Retrieved 2009-06-06.
- ^ R. P. MacDonald (1964). "Uses for a Holmium Oxide Filter in Spectrophotometry" (PDF). Clinical Chemistry. 10: 1117.
- ^ Travis, John C.; Zwinkels, JC; Mercader, F; Ruíz, A; Early, EA; Smith, MV; Noël, M; Maley, M; Kramer, GW (2002). "An International Evaluation of Holmium Oxide Solution Reference Materials for Wavelength Calibration in Molecular Absorption Spectrophotometry". Analytical Chemistry. 74 (14): 3408. doi:10.1021/ac0255680. PMID 12139047.
- ^ "Holmium Glass Filter for Spectrophotometer Calibration". Retrieved 2009-06-06.
- ^ Yehoshua Y. Kalisky (2006). The physics and engineering of solid state lasers. SPIE Press. p. 125. ISBN 081946094X.
- ^ "External MSDS" (PDF). Retrieved 2009-06-06.
