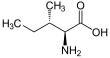
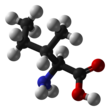
Isoleucine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Isoleucine
| |||
| Other names
2-Amino-3-methylpentanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.726 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H13NO2 | |||
| Molar mass | 131.175 g·mol−1 | ||
| Supplementary data page | |||
| Isoleucine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Isoleucine (abbreviated as Ile or I) encoded by the codons ATT, ATC, ATA[1] is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain, classifying it as a non-polar, uncharged(at physiological pH), aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria.[2]
Inability to break down isoleucine, along with other amino acids, is associated with the disease called Maple Syrup Urine Disease, which results in discoloration and a sweet smell in the patient's urine, which is where the name comes from. However, in severe cases, MSUD can lead to damage to the brain cells and ultimately death.[3]
Metabolism
Biosynthesis
As an essential nutrient, it is not synthesized in the body, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, starting from pyruvic acid and alpha-ketoglutarate. Enzymes involved in this biosynthesis include:[4]
- Acetolactate synthase (also known as acetohydroxy acid synthase)
- Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- Valine aminotransferase
Catabolism
Isoleucine is both a glucogenic and a ketogenic amino acid. After transamination with alpha-ketoglutarate the carbon skeleton can be converted into either Succinyl CoA, and fed into the TCA cycle for oxidation or converted into oxaloacetate for gluconeogenesis (hence glucogenic). It can also be converted into Acetyl CoA and fed into the TCA cycle by condensing with oxaloacetate to form citrate. In mammals Acetyl CoA cannot be converted back to carbohydrate but can be used in the synthesis of ketone bodies or fatty acids, hence ketogenic.
Biotin, sometimes referred to as Vitamin B7 or Vitamin H, is an absolute requirement for the full catabolism of isoleucine (as well as leucine). Without adequate biotin, the human body will be unable to fully break down isoleucine and leucine molecules.[5]
Nutritional Sources
Even though this amino acid is not produced in animals, it is stored in high quantities. Foods that have high amounts of isoleucine include eggs, soy protein, seaweed, turkey, chicken, lamb, cheese, and fish.[6]
Isomers of isoleucine
| Forms of Isoleucine | |||||||
|---|---|---|---|---|---|---|---|
| Common name: | isoleucine | D-isoleucine | L-isoleucine | DL-isoleucine | allo-D-isoleucine | allo-L-isoleucine | allo-DL-isoleucine |
| Synonyms: | (R)-Isoleucine | L(+)-Isoleucine | (R*,R*)-isoleucine | alloisoleucine | |||
| PubChem: | CID 791 from PubChem | CID 94206 from PubChem | CID 6306 from PubChem | CID 76551 from PubChem | |||
| EINECS number: | |||||||
| CAS number: | 443-79-8 | 319-78-8 | 73-32-5 | 1509-35-9 | 1509-34-8 | 3107-04-8 | |
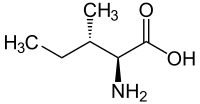  |
| L-isoleucine (2S,3S) and D-isoleucine (2R,3R) |
  |
| L-allo-isoleucine (2S,3R) and D-allo-isoleucine (2R,3S) |
Synthesis
Isoleucine can be synthesized in a multistep procedure starting from 2-bromobutane and diethylmalonate.[7] Synthetic isoleucine was originally reported in 1905.[8]
German chemist Felix Ehrlich discovered isoleucine in hemoglobin in 1903.
References
- ^ Center for Biological Sequence Analysis, University of Denmark http://www.cbs.dtu.dk/courses/27619/codon.html
- ^ Kisumi, M; Komatsubara, S; Chibata, I (Jul 1977). "Pathway for isoleucine formation form pyruvate by leucine biosynthetic enzymes in leucine-accumulating isoleucine revertants of Serratia marcescens". J. Biochem. PMID 142769.
- ^ "Maple Syrup Urine Disease (MSUD)". learn.genetics.utah.edu. Retrieved 2015-12-08.
- ^ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ^ "Biotin & Detoxification Needs in Cognitively Delayed Adult - Metametrix Learning Center". Metametrix.com. Retrieved 2013-10-18.
- ^ [1], List is in order of highest to lowest of per 200 Calorie serving of the food, not volume or weight.
- ^ "dl-Isoleucine". Organic Syntheses. 1955; Collected Volumes, vol. 3, p. 495.
- ^ Bouveault and Locquin, Compt. rend., 141, 115 (1905).
Center for Biological Sequence Analysis, University of Denmark http://www.cbs.dtu.dk/courses/27619/codon.html