Leukotriene E4

| |
| Names | |
|---|---|
| Systematic IUPAC name
(5S,6R,7E,9E,11Z,14Z)-6-({(2R)-2-Amino-2-carboxyethyl}sulfanyl)-5-hydroxyicosa-7,9,11,14-tetraenoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | LTE4 |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | Leukotriene+E4 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H37NO5S | |
| Molar mass | 439.61 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
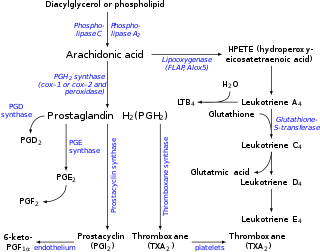
Leukotriene E4 (LTE4) is a cysteinyl leukotriene involved in inflammation. It is known to be produced by several types of white blood cells, including eosinophils, mast cells, tissue macrophages, and basophils, and recently was also found to be produced by platelets adhering to neutrophils.[1] It is formed from the sequential conversion of LTC4 to LTD4 and then to LTE4, which is the final and most stable cysteinyl leukotriene.[2] Compared to the short half lives of LTC4 and LTD4, LTE4 is relatively stable and accumulates in breath condensation, in plasma, and in urine, making it the dominant cysteinyl leukotriene detected in biologic fluids.[3] Therefore, measurements of LTE4, especially in the urine, are commonly monitored in clinical research studies.
Increased production and excretion of LTE4 has been linked to several respiratory diseases, and urinary LTE4 levels are increased during severe asthma attacks and are especially high in people with aspirin-exacerbated respiratory disease.[4]
Studies have suggested that LTE4 works through its own distinct receptor, and although one has not yet been discovered, research is ongoing to isolate and characterize an LTE4-specific receptor.[5][6]
References
[edit]- ^ Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, Castells MC, Chhay H, Boyce JA (2012). "Cysteinyl leukotriene overproduction in aspirin exacerbated respiratory disease is driven by platelet-adherent leukocytes". Blood. 119 (16): 3790–3798. doi:10.1182/blood-2011-10-384826. PMC 3335383. PMID 22262771.
- ^ Lee CW, Lewis RA, Corey EJ, Austen KF (1983). "Conversion of leukotriene D4 to leukotriene E4 by a dipeptidase released from the specific granule of human polymorphonuclear leucocytes". Immunology. 48 (1): 27–35. PMC 1453997. PMID 6293969.
- ^ Sala A, Voelkel N, Maclouf J, Murphy RC (1990). "Leukotriene E4 elimination and metabolism in normal human subjects". J Biol Chem. 265 (35): 21771–21778. doi:10.1016/S0021-9258(18)45807-3. hdl:2434/180227. PMID 2174886.
- ^ Lee TH, Christie PE (1993). "Leukotrienes and aspirin induced asthma". Thorax. 48 (12): 1189–1190. doi:10.1136/thx.48.12.1189. PMC 464963. PMID 8303620.
- ^ Maekawa A, Kanaoka Y, Xing W; Kanaoka; Xing; Austen (2008). "Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors". Proc. Natl. Acad. Sci. USA. 105 (43): 16695–16700. Bibcode:2008PNAS..10516695M. doi:10.1073/pnas.0808993105. PMC 2575482. PMID 18931305.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Paruchuri, S; Tashimo, H; Feng, C; Maekawa, A; Xing, W; Jiang, Y; Kanaoka, Y; Conley, P; Boyce, JA (2009). "Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor". The Journal of Experimental Medicine. 206 (11): 2543–55. doi:10.1084/jem.20091240. PMC 2768854. PMID 19822647.
