Non-mevalonate pathway
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP).[1][2][3] The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP.
Isoprenoid precursor biosynthesis
The classical mevalonate pathway or HMG-CoA reductase pathway is a metabolic pathway from the biosynthesis of isoprenoid precursors present in most higher eukaryotes and some bacteria.[citation needed] It is important for the production of IPP and DMAPP, which serve as the basis for the biosynthesis of isoprenoid (terpenoid) molecules used in processes as diverse as protein prenylation, cell membrane maintenance, the synthesis of hormones, protein anchoring and N-glycosylation.[citation needed]
Bacteria, plants, and apicomplexan protozoa—such as malaria parasites—are able to produce isoprenoid precursors using an alternative pathway, the MEP pathway, which is a non-mevalonate pathway. In the case of plants and certain protozoa, the biosynthesis of IPP/DMAPP takes place in plastid organelles.[4] Plants synthesise isoprenoid precursors using the mevalonate pathway in the cytoplasm and using the MEP pathway in their chloroplasts. Bacteria that use the pathway include important pathogens such Mycobacterium tuberculosis.[5]
Reactions
This section relies largely or entirely on a single source. (March 2017) |
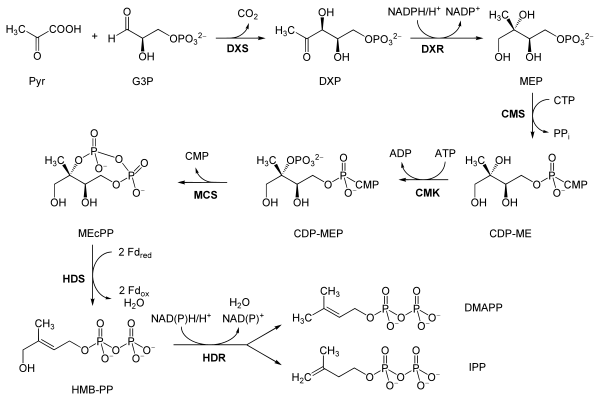
The reactions of the non-mevalonate pathway are as follows, taken primarily from Eisenreich and co-workers, except where the bold labels are additional local abbreviations to assist in connecting the table to the scheme above:[7][6]
| Reactants | Enzyme | Product | |
| Pyruvate (Pyr) and glyceraldehyde 3-phosphate (G3P) | DOXP synthase (Dxs; DXR) | 1-Deoxy-D-xylulose 5-phosphate (DOXP; DXP) |  |
| DOXP (DXP) | DXP reductoisomerase (Dxr, IspC; DXR) | 2-C-methylerythritol 4-phosphate (MEP) |  |
| MEP | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase (YgbP, IspD; CMS) | 4-diphosphocytidyl-2-C-methylerythritol (CDP-ME) |  |
| CDP-ME | 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase (YchB, IspE; CMK) | 4-diphosphocytidyl-2-C-methyl-D-erythritol 2-phosphate (CDP-MEP) | |
| CDP-MEP | 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (YgbB, IspF; MCS) | 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) |  |
| MEcPP | HMB-PP synthase (GcpE, IspG; HDS) | (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) |  |
| HMB-PP | HMB-PP reductase (LytB, IspH; HDR) | Isopentenyl pyrophosphate (IPP) and Dimethylallyl pyrophosphate (DMAP) |   |
Inhibition and other pathway research
DXP reductoisomerase (also known as: DXR, DOXP reductoisomerase, IspC, MEP synthase), is a key enzyme in the MEP pathway. It can be inhibited by the natural product fosmidomycin, which is under study as a starting point to develop a candidate antibacterial or antimalarial drug.[8][9][10]
The intermediate, HMB-PP, is a natural activator of human Vγ9/Vδ2 T cells, the major γδ T cell population in peripheral blood, and cells that "play a crucial role in the immune response to microbial pathogens".[11]
References
- ^ Rohmer M; Rohmer, Michel (1999). "The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants". Nat Prod Rep. 16 (5): 565–574. doi:10.1039/a709175c. PMID 10584331.
- ^ W. Eisenreich; A. Bacher; D. Arigoni; F. Rohdich (2004). "Review Biosynthesis of isoprenoids via the non-mevalonate pathway". Cellular and Molecular Life Sciences. 61 (12): 1401–1426. doi:10.1007/s00018-004-3381-z. PMID 15197467.
- ^ Hunter, WN (2007). "The Non-mevalonate Pathway of Isoprenoid Precursor Biosynthesis". Journal of Biological Chemistry. 282 (30): 21573–21577. doi:10.1074/jbc.R700005200. Retrieved March 23, 2017.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lichtenthaler H (1999). "The 1-Deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants". Annu Rev Plant Physiol Plant Mol Biol. 50: 47–65. doi:10.1146/annurev.arplant.50.1.47. PMID 15012203.
- ^ Wiemer, AJ; Hsiao, CH; Wiemer, DF (2010). "Isoprenoid Metabolism as a Therapeutic Target in Gram-Negative Pathogens". Current Topics in Medicinal Chemistry. 10 (18): 1858–1871. doi:10.2174/156802610793176602. PMID 20615187.
- ^ a b "Exploring Drug Targets in Isoprenoid Biosynthetic Pathway for Plasmodium falciparum". Biochemistry Research International. 2014: 657189. 2014. doi:10.1155/2014/657189. PMC 4017727. PMID 24864210.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link) - ^ a b Eisenreich W, Bacher A, Arigoni D, Rohdich F (2004). "Biosynthesis of Isoprenoids Via the Non-mevalonate Pathway". Cell. Mol. Life Sci. 61 (12): 1401–26. doi:10.1007/s00018-004-3381-z. PMID 15197467.
- ^ "The MEP pathway and the Development of Inhibitors as Potential Anti-Infective Agents" (review). Med. Chem. Commun. 3: 418–433. 2012. doi:10.1039/C2MD00298A. Retrieved March 23, 2017.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Jomaa H, Wiesner J, Sanderbrand S, et al. (1999). "Inhibitors of the Nonmevalonate Pathway of Isoprenoid Biosynthesis as Antimalarial Drugs". Science. 285 (5433): 1573–6. doi:10.1126/science.285.5433.1573. PMID 10477522.
{{cite journal}}:|format=requires|url=(help) - ^ C. Zinglé; L. Kuntz; D. Tritsch; C. Grosdemange-Billiard; M. Rohmer (2010). "Isoprenoid Biosynthesis via the Methylerythritol Phosphate Pathway: Structural Variations around Phosphonate Anchor and Spacer of Fosmidomycin, a Potent Inhibitor of Deoxyxylulose Phosphate Reductoisomerase". J. Org. Chem. 75 (10): 3203–3207. doi:10.1021/jo9024732.
{{cite journal}}:|format=requires|url=(help) - ^ Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H (2003). "Microbial Isoprenoid Biosynthesis and Human γδ T cell Activation" (minireview). FEBS Lett. 544 (1–3): 4–10. doi:10.1016/S0014-5793(03)00483-6. PMID 12782281. Retrieved March 23, 2017.
Further reading
- "The MEP pathway and the Development of Inhibitors as Potential Anti-Infective Agents" (review). Med. Chem. Commun. 3: 418–433. 2012. doi:10.1039/C2MD00298A. Retrieved March 23, 2017.
{{cite journal}}: Unknown parameter|authors=ignored (help) RSC review; uses MAP synthase nomenclature. - "Exploring Drug Targets in Isoprenoid Biosynthetic Pathway for Plasmodium falciparum". Biochemistry Research International. 2014: 657189. 2014. doi:10.1155/2014/657189. PMC 4017727. PMID 24864210.
{{cite journal}}: Unknown parameter|authors=ignored (help)CS1 maint: unflagged free DOI (link)