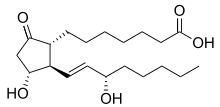
Prostaglandin E1
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.925 |
| Chemical and physical data | |
| Formula | C20H34O5 |
| Molar mass | 354.481 g/mol g·mol−1 |
Alprostadil is the pharmaceutical name for prostaglandin E1. It is used as a drug in the treatment of erectile dysfunction and has vasodilatory properties.[2]
Sexual dysfunction
Aprostidil is sold in the United States under the brand name Muse.[3] It is also sold as Caverject and Edex.
Muse delivers alprostadil as a penile suppository, inserted into the urethra, at least ten minutes before the erection will be needed. Caverject and Edex are similarly fast-acting, but are actually injected directly into the corpus cavernosum of the penis.
This drug is reputed to work for erectile dysfunction for 30 to 60 minutes.
This drug, following on the coattails of Viagra, has failed to achieve comparable above-ground market share in the United States, perhaps in part because of the invasive means of delivery.
NexMed is developing transdermal forumulations—Alprox-TD(R) for men with erectile dysfunction and Femprox(R) for female sexual arousal disorder. Alprox-TD(R) has been selling in China and in Hong Kong since October 2001 and April 2002, respectively. Two Phase III studies have been completed for this product, and the Company is seeking regulatory approval in the U.S., Canada and Europe. [4]
Other uses
Aprostadil is also used in maintaining a patent ductus arteriosus in the fetus. This is primarily useful when there is threat of premature closure of the ductus arteriosus.[2]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b "12123006" at Dorland's Medical Dictionary
- ^ Muse.
- ^ Fain Hughes (2007-10-29). "NEXM: Dutton Sees Strong Speculative Buy and 12-Month Price Double". Retrieved 2007-11-01.
