Proton
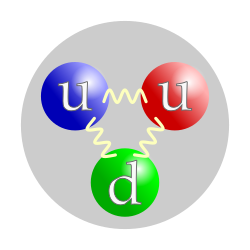 The quark structure of the proton. (The color assignment of individual quarks is not important, only that all three colors are present.) | |
| Classification | Baryon |
|---|---|
| Composition | 2 up quarks, 1 down quark |
| Statistics | Fermionic |
| Family | Hadron |
| Interactions | Gravity, Electromagnetic, Weak, Strong |
| Symbol | p , p+ , N+ |
| Antiparticle | Antiproton |
| Theorized | William Prout (1815) |
| Discovered | Ernest Rutherford (1917–1919, named by him, 1920) |
| Mass | 1.672621777(74)×10−27 kg[1] 1.007276466812(90) u[1] |
| Mean lifetime | >2.1×1029 years (stable) |
| Electric charge | +1 e 1.602176565(35)×10−19 C[1] |
| Charge radius | 0.8775(51) fm[1] |
| Electric dipole moment | <5.4×10−24 e·cm |
| Electric polarizability | 1.20(6)×10−3 fm3 |
| Magnetic moment | 1.410606743(33)×10−26 J·T−1[1] 2.792847356(23) μN[1] |
| Magnetic polarizability | 1.9(5)×10−4 fm3 |
| Spin | 1⁄2 |
| Isospin | 1⁄2 |
| Parity | +1 |
| Condensed | I(JP) = 1⁄2(1⁄2+) |
The proton is a subatomic particle with the symbol
p
or
p+
and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom. The number of protons in each atom is its atomic number. The name proton was given to the hydrogen nucleus by Ernest Rutherford in 1920, because in previous years he had discovered that the hydrogen nucleus (known to be the lightest nucleus) could be extracted from the nuclei of nitrogen by collision, and was thus a candidate to be a fundamental particle and building block of nitrogen, and all other heavier atomic nuclei.
In the modern Standard Model of particle physics, the proton is a hadron, composed of quarks. Prior to that model becoming a consensus in the physics community, the proton was considered a fundamental particle. In the modern view, a proton is composed of three valence quarks: two up quarks and one down quark. The rest masses of the quarks are thought to contribute only about 1% of the proton's mass. The remainder of the proton mass is due to the kinetic energy of the quarks and to the energy of the gluon fields that bind the quarks together.
Because the proton is not a fundamental particle, it possesses a physical size—although this is not perfectly well-defined since the surface of a proton is somewhat fuzzy, due to being defined by the influence of forces that do not come to an abrupt end. The proton is about 1.6–1.7 fm in diameter.[3]
The free proton (a proton not bound to nucleons or electrons) is a stable particle that has not been observed to break down spontaneously to other particles. Free protons are found naturally in a number of situations in which energies or temperatures are high enough to separate them from electrons, for which they have some affinity. Free protons exist in plasmas in which temperatures are too high to allow them to combine with electrons. Free protons of high energy and velocity make up 90% of cosmic rays, which propagate in vacuum for interstellar distances. Free protons are emitted directly from atomic nuclei in some rare types of radioactive decay. Protons also result (along with electrons and antineutrinos) from the radioactive decay of free neutrons, which are unstable.
At sufficiently low temperatures, free protons will bind to electrons. However, the character of such bound protons does not change, and they remain protons. A fast proton moving through matter will slow by interactions with electrons and nuclei, until it is captured by the electron cloud of an atom. The result is a protonated atom, which is a chemical compound of hydrogen. In vacuum, when free electrons are present, a sufficiently slow proton may pick up a single free electron, becoming a neutral hydrogen atom, which is chemically a free radical. Such "free hydrogen atoms" tend to react chemically with many other types of atoms at sufficiently low energies. When free hydrogen atoms react with each other, they form neutral hydrogen molecules (H2), which are the most common molecular component of molecular clouds in interstellar space. Such molecules of hydrogen on Earth may then serve (among many other uses) as a convenient source of protons for accelerators (as used in proton therapy) and other hadron particle physics experiments that require protons to accelerate, with the most powerful and noted example being the large hadron collider.
Description
Protons are spin-½ fermions and are composed of three valence quarks,[4] making them baryons (a sub-type of hadrons). The two up quarks and one down quark of the proton are held together by the strong force, mediated by gluons.[3] A modern perspective has the proton composed of the valence quarks (up, up, down), the gluons, and transitory pairs of sea quarks. The proton has an approximately exponentially decaying positive charge distribution with a mean square radius of about 0.8 fm.[5]
Protons and neutrons are both nucleons, which may be bound by the nuclear force into atomic nuclei. The nucleus of the most common isotope of the hydrogen atom (with the chemical symbol "H") is a lone proton. The nuclei of the heavy hydrogen isotopes deuterium and tritium contain one proton bound to one and two neutrons, respectively. All other types of atoms are composed of two or more protons and various numbers of neutrons.
Stability
The spontaneous decay of free protons has never been observed, and the proton is therefore considered a stable particle. However, some grand unified theories of particle physics predict that proton decay should take place with lifetimes of the order of 1036 years, and experimental searches have established lower bounds on the mean lifetime of the proton for various assumed decay products.
Experiments at the Super-Kamiokande detector in Japan gave lower limits for proton mean lifetime of 6.6×1033 years for decay to an antimuon and a neutral pion, and 8.2×1033 years for decay to a positron and a neutral pion.[6] Another experiment at the Sudbury Neutrino Observatory in Canada searched for gamma rays resulting from residual nuclei resulting from the decay of a proton from oxygen-16. This experiment was designed to detect decay to any product, and established a lower limit to the proton lifetime of 2.1×1029 years.[7]
However, protons are known to transform into neutrons through the process of electron capture (also called inverse beta decay). For free protons, this process does not occur spontaneously but only when energy is supplied. The equation is:
The process is reversible; neutrons can convert back to protons through beta decay, a common form of radioactive decay. In fact, a free neutron decays this way, with a mean lifetime of about 15 minutes.
Quarks and the mass of the proton
In quantum chromodynamics, the modern theory of the nuclear force, most of the mass of the proton and the neutron is explained by special relativity. The mass of the proton is about 80-100 times greater than the sum of the rest masses of the quarks that make it up, while the gluons have zero rest mass. The extra energy of the quarks and gluons in a region within a proton, as compared to the rest energy of the quarks alone in the QCD vacuum, accounts for almost 99% of the mass. The rest mass of the proton is, thus, the invariant mass of the system of moving quarks and gluons that make up the particle, and, in such systems, even the energy of massless particles is still measured as part of the rest mass of the system.
Two terms are used in referring to the mass of the quarks that make up protons: Current quark mass refers to the mass of a quark by itself, while constituent quark mass refers to the current quark mass plus the mass of the gluon particle field surrounding the quark.[8] These masses typically have very different values. As noted, most of a proton's mass comes from the gluons that bind the constituent quarks together, rather than from the quarks themselves. While gluons are inherently massless, they possess energy— to be more specific, quantum chromodynamics binding energy (QCBE)—and it is this that contributes so greatly to the overall mass of the proton (see mass in special relativity). A proton has a mass of approximately 938 MeV/c2, of which the rest mass of its three valence quarks contributes only about 11 MeV/c2; much of the remainder can be attributed to the gluons' QCBE.[9]
The internal dynamics of the proton are complicated, because they are determined by the quarks' exchanging gluons, and interacting with various vacuum condensates. Lattice QCD provides a way of calculating the mass of the proton directly from the theory to any accuracy, in principle. The most recent calculations[10][11] claim that the mass is determined to better than 4% accuracy, even to 1% accuracy (see Figure S5 in Dürr et al.[11]). These claims are still controversial, because the calculations cannot yet be done with quarks as light as they are in the real world. This means that the predictions are found by a process of extrapolation, which can introduce systematic errors.[12] It is hard to tell whether these errors are controlled properly, because the quantities that are compared to experiment are the masses of the hadrons, which are known in advance.
These recent calculations are performed by massive supercomputers, and, as noted by Boffi and Pasquini: "a detailed description of the nucleon structure is still missing because ... long-distance behavior requires a nonperturbative and/or numerical treatment..."[13] More conceptual approaches to the structure of the proton are: the topological soliton approach originally due to Tony Skyrme and the more accurate AdS/QCD approach that extends it to include a string theory of gluons, various QCD-inspired models like the bag model and the constituent quark model, which were popular in the 1980s, and the SVZ sum rules, which allow for rough approximate mass calculations. These methods do not have the same accuracy as the more brute-force lattice QCD methods, at least not yet.
Charge radius
The internationally-accepted value of the proton's charge radius is 0.8768 fm (see orders of magnitude for comparison to other sizes). This value is based on measurements involving a proton and an electron.
However, since July 5, 2010, an international research team has been able to make measurements involving a proton and a negatively-charged muon. After a long and careful analysis of those measurements, the team concluded that the root-mean-square charge radius of a proton is "0.84184(67) fm, which differs by 5.0 standard deviations from the CODATA value of 0.8768(69) fm."[14]
The international research team that obtained this result at the Paul Scherrer Institut (PSI) in Villigen (Switzerland) includes scientists from the Max Planck Institute of Quantum Optics (MPQ) in Garching, the Ludwig-Maximilians-Universität (LMU) Munich and the Institut für Strahlwerkzeuge (IFWS) of the Universität Stuttgart (both from Germany), and the University of Coimbra, Portugal.[15][16] They are now attempting to explain the discrepancy, and re-examining the results of both previous high-precision measurements and complicated calculations. If no errors are found in the measurements or calculations, it could be necessary to re-examine the world’s most precise and best-tested fundamental theory: quantum electrodynamics.[17]
In the experiment described in recent published Science article (January 2013), the same research team report charge radius of exotic hydrogen (involving a proton and a negatively-charged muon) is 0.84087(39) fm.[18]
Interaction of free protons with ordinary matter
Although protons have affinity for oppositely-charged electrons, free protons must lose sufficient velocity (and kinetic energy) in order to become closely associated and bound to electrons, since this is a relatively low-energy interaction. High energy protons, in traversing ordinary matter, lose energy by collisions with atomic nuclei, and by ionization of atoms (removing electrons) when until they are slowed sufficiently to be captured by the electron cloud in a normal atom.
However, in such an association with an electron, the character of the bound proton is not changed, and it remains a proton. The attraction of low-energy free protons to any electrons present in normal matter (such as the electrons in normal atoms) causes free protons to stop and to form a new chemical bond with an atom. Such a bond happens at any sufficiently "cold" temperature (i.e., comparable to temperatures at the surface of the Sun) and with any type of atom. Thus, in interaction with any type of normal (non-plasma) matter, low-velocity free protons are attracted to electrons in any atom or molecule with which they come in contact, causing the proton and molecule to combine. Such molecules are then said to be "protonated," and chemically they often, as a result, become so-called Bronsted acids.
Proton in chemistry
Atomic number
In chemistry, the number of protons in the nucleus of an atom is known as the atomic number, which determines the chemical element to which the atom belongs. For example, the atomic number of chlorine is 17; this means that each chlorine atom has 17 protons and that all atoms with 17 protons are chlorine atoms. The chemical properties of each atom are determined by the number of (negatively charged) electrons, which for neutral atoms is equal to the number of (positive) protons so that the total charge is zero. For example, a neutral chlorine atom has 17 protons and 17 electrons, whereas a Cl− anion has 17 protons and 18 electrons for a total charge of −1.
All atoms of a given element are not necessarily identical, however, as the number of neutrons may vary to form different isotopes, and energy levels may differ forming different nuclear isomers. For example, there are two stable isotopes of chlorine: 35
17Cl
with 35 − 17 = 18 neutrons and 37
17Cl
with 37 − 17 = 20 neutrons.
Hydrogen ion
In chemistry, the term proton refers to the hydrogen ion, H+
. Since the atomic number of hydrogen is 1, a hydrogen ion has no electrons and corresponds to a bare nucleus, consisting of a proton (and 0 neutrons for the most abundant isotope protium 1
1H
). The proton is a "bare charge" with only about 1/64,000 of the radius of a hydrogen atom, and so is extremely reactive chemically. The free proton, thus, has an extremely short lifetime in chemical systems such as liquids and it reacts immediately with the electron cloud of any available molecule. In aqueous solution, it forms the hydronium ion, H3O+, which in turn is further solvated by water molecules in clusters such as [H5O2]+ and [H9O4]+.[19]
The transfer of H+
in an acid–base reaction is usually referred to as "proton transfer". The acid is referred to as a proton donor and the base as a proton acceptor. Likewise, biochemical terms such as proton pump and proton channel refer to the movement of hydrated H+
ions.
The ion produced by removing the electron from a deuterium atom is known as a deuteron, not a proton. Likewise, removing an electron from a tritium atom produces a triton.
Proton nuclear magnetic resonance (NMR)
Also in chemistry, the term "proton NMR" refers to the observation of hydrogen-1 nuclei in (mostly organic) molecules by nuclear magnetic resonance. This method uses the spin of the proton, which has the value one-half. The name refers to examination of protons as they occur in protium (hydrogen-1 atoms) in compounds, and does not imply that free protons exist in the compound being studied.
History
The concept of a hydrogen-like particle as a constituent of other atoms was developed over a long period. As early as 1815, William Prout proposed that all atoms are composed of hydrogen atoms (which he called "protyles"), based on a simplistic interpretation of early values of atomic weights (see Prout's hypothesis), which was disproved when more accurate values were measured.

In 1886, Eugen Goldstein discovered canal rays (also known as anode rays) and showed that they were positively charged particles (ions) produced from gases. However, since particles from different gases had different values of charge-to-mass ratio (e/m), they could not be identified with a single particle, unlike the negative electrons discovered by J. J. Thomson.
Following the discovery of the atomic nucleus by Ernest Rutherford in 1911, Antonius van den Broek proposed that the place of each element in the periodic table (its atomic number) is equal to its nuclear charge. This was confirmed experimentally by Henry Moseley in 1913 using X-ray spectra.
In 1917, (in experiments reported in 1919) Rutherford proved that the hydrogen nucleus is present in other nuclei, a result usually described as the discovery of the proton.[20] Rutherford had earlier learned to produce hydrogen nuclei as a type of radiation produced as a product of the impact of alpha particles on hydrogen gas, and recognize them by their unique penetration signature in air and their appearance in scintillation detectors. These experiments were begun when Rutherford had noticed that, when alpha particles were shot into air (mostly nitrogen), his scintillation detectors showed the signatures of typical hydrogen nuclei as a product. After experimentation Rutherford traced the reaction to the nitrogen in air, and found that when alphas were produced into pure nitrogen gas, the effect was larger. Rutherford determined that this hydrogen could have come only from the nitrogen, and therefore nitrogen must contain hydrogen nuclei. One hydrogen nucleus was being knocked off by the impact of the alpha particle, producing oxygen-17 in the process. This was the first reported nuclear reaction, 14N + α → 17O + p. (This reaction would later be observed happening directly in a cloud chamber in 1925).
Rutherford knew hydrogen to be the simplest and lightest element and was influenced by Prout's hypothesis that hydrogen was the building block of all elements. Discovery that the hydrogen nucleus is present in all other nuclei as an elementary particle, led Rutherford to give the hydrogen nucleus a special name as a particle, since he suspected that hydrogen, the lightest element, contained only one of these particles. He named this new fundamental building block of the nucleus the proton, after the neuter singular of the Greek word for "first", πρῶτον. However, Rutherford also had in mind the word protyle as used by Prout. Rutherford spoke at the British Association for the Advancement of Science at its Cardiff meeting beginning August 24, 1920.[21] Rutherford was asked by Oliver Lodge for a new name for the positive hydrogen nucleus to avoid confusion with the neutral hydrogen atom. He initially suggested both proton and prouton (after Prout).[22] Rutherford later reported that the meeting had accepted his suggestion that the hydrogen nucleus be named the "proton," following Prout's word "protyle."[23] The first use of the word "proton" in the scientific literature appeared in 1920.[24]
Exposure
The Apollo Lunar Surface Experiments Packages (ALSEP) determined that more than 95% of the particles in the solar wind are electrons and protons, in approximately equal numbers.[25][26]
Because the Solar Wind Spectrometer made continuous measurements, it was possible to measure how the Earth's magnetic field affects arriving solar wind particles. For about two-thirds of each orbit, the Moon is outside of the Earth's magnetic field. At these times, a typical proton density was 10 to 20 per cubic centimeter, with most protons having velocities between 400 and 650 kilometers per second. For about five days of each month, the Moon is inside the Earth's geomagnetic tail, and typically no solar wind particles were detectable. For the remainder of each lunar orbit, the Moon is in a transitional region known as the magnetosheath, where the Earth's magnetic field affects the solar wind but does not completely exclude it. In this region, the particle flux is reduced, with typical proton velocities of 250 to 450 kilometers per second. During the lunar night, the spectrometer was shielded from the solar wind by the Moon and no solar wind particles were measured.[25]
Protons also occur in from extrasolar origin in space, from galactic cosmic rays, where they make up about 90% of the total particle flux. These protons often have higher energy than solar wind protons, but their intensity is far more uniform and less variable than protons coming from the Sun, the production of which is heavily affected by solar proton events such as coronal mass ejections.
Research has been performed on the dose-rate effects of protons, as typically found in space travel, on human health.[26][27] To be more specific, there are hopes to identify what specific chromosomes are damaged, and to define the damage, during cancer development from proton exposure.[26] Another study looks into determining "the effects of exposure to proton irradiation on neurochemical and behavioral endpoints, including dopaminergic functioning, amphetamine-induced conditioned taste aversion learning, and spatial learning and memory as measured by the Morris water maze."[27] Electrical charging of a spacecraft due to interplanetary proton bombardment has also been proposed for study.[28] There are many more studies that pertain to space travel, including galactic cosmic rays and their possible health effects, and solar proton event exposure.
The American Biostack and Soviet Biorack space travel experiments have demonstrated the severity of molecular damage induced by heavy ions on micro organisms including Artemia cysts.[29]
Antiproton
CPT-symmetry puts strong constraints on the relative properties of particles and antiparticles and, therefore, is open to stringent tests. For example, the charges of the proton and antiproton must sum to exactly zero. This equality has been tested to one part in 108. The equality of their masses has also been tested to better than one part in 108. By holding antiprotons in a Penning trap, the equality of the charge to mass ratio of the proton and the antiproton has been tested to one part in 6×109.[30] The magnetic moment of the antiproton has been measured with error of 8×10−3 nuclear Bohr magnetons, and is found to be equal and opposite to that of the proton.
See also
References
- ^ a b c d e f g h P.J. Mohr, B.N. Taylor, and D.B. Newell (2011), "The 2010 CODATA Recommended Values of the Fundamental Physical Constants" (Web Version 6.0). This database was developed by J. Baker, M. Douma, and S. Kotochigova. Available: http://physics.nist.gov/constants [Thursday, 02-Jun-2011 21:00:12 EDT]. National Institute of Standards and Technology, Gaithersburg, MD 20899.
- ^
B.P. Stein, R. Ladbury, P.F. Schewe (1995). "Physics Update". Physics Today. 48 (10): 9. Bibcode:1995PhT....48j...9S. doi:10.1063/1.2808195.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b W.N. Cottingham, D.A. Greenwood (1986). An Introduction to Nuclear Physics. Cambridge University Press. p. 19.
- ^ R.K. Adair (1989). The Great Design: Particles, Fields, and Creation. Oxford University Press. p. 214.
- ^
J.-L. Basdevant, J. Rich, M. Spiro (2005). Fundamentals in Nuclear Physics. Springer. p. 155. ISBN 0-387-01672-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^
H. Nishino et al. (Kamiokande collaboration) (2009). "Search for Proton Decay via
p
→
e+
π0
and
p
→
μ+
π0
in a Large Water Cherenkov Detector". Physical Review Letters. 102 (14): 141801. arXiv:0903.0676. Bibcode:2009PhRvL.102n1801N. doi:10.1103/PhysRevLett.102.141801. - ^ S.N. Ahmed et al. (SNO Collaboration) (2004). "Constraints on nucleon decay via invisible modes from the Sudbury Neutrino Observatory". Physical Review Letters. 92 (10): 102004. arXiv:hep-ex/0310030. Bibcode:2004PhRvL..92j2004A. doi:10.1103/PhysRevLett.92.102004. PMID 15089201.
- ^ A. Watson (2004). The Quantum Quark. Cambridge University Press. pp. 285–286. ISBN 0-521-82907-0.
- ^ W. Weise, A.M. Green (1984). Quarks and Nuclei. World Scientific. pp. 65–66. ISBN 9971-966-61-1.
- ^ See this news report and links
- ^ a b S. Dürr, Z. Fodor, J. Frison, C. Hoelbling, R. Hoffmann, S. D. Katz, S. Krieg, T. Kurth, L. Lellouch, T. Lippert, K. K. Szabo, and G. Vulvert (21 November 2008). "Ab Initio Determination of Light Hadron Masses". Science. 322 (5905): 1224. arXiv:0906.3599. Bibcode:2008Sci...322.1224D. doi:10.1126/science.1163233. PMID 19023076.
{{cite journal}}: More than one of|pages=and|page=specified (help)CS1 maint: multiple names: authors list (link) - ^ C. F. Perdrisat, V. Punjabi, M. Vanderhaeghen (2007). "Nucleon Electromagnetic Form Factors". Prog Part Nucl Phys. 59 (2): 694–764. arXiv:hep-ph/0612014. Bibcode:2007PrPNP..59..694P. doi:10.1016/j.ppnp.2007.05.001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sigfrido Boffi & Barbara Pasquini (2007). "Generalized parton distributions and the structure of the nucleon". Rivista del Nuovo Cimento. 30. arXiv:0711.2625. Bibcode:2007NCimR..30..387B. doi:10.1393/ncr/i2007-10025-7.
- ^ Randolf Pohl, Aldo Antognini, François Nez, Fernando D. Amaro, François Biraben, João M. R. Cardoso, Daniel S. Covita, Andreas Dax, Satish Dhawan, Luis M. P. Fernandes, Adolf Giesen, Thomas Graf, Theodor W. Hänsch, Paul Indelicato, Lucile Julien, Cheng-Yang Kao, Paul Knowles, Eric-Olivier Le Bigot, Yi-Wei Liu, José A. M. Lopes, Livia Ludhova, Cristina M. B. Monteiro, Françoise Mulhauser, Tobias Nebel, Paul Rabinowitz; et al. (8 July 2010). "The size of the proton". Nature. 466 (7303): 213–216. Bibcode:2010Natur.466..213P. doi:10.1038/nature09250. PMID 20613837. Retrieved 2010-07-09.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ New proton measurements may throw physics a curve
- ^ "The Proton Just Got Smaller". Photonics.Com. July 12, 2010. Retrieved 2010-07-19.
- ^ Researchers Observes Unexpectedly Small Proton Radius in a Precision Experiment
- ^ Aldo Antognini; et al. (25 January 2013). "Proton Structure from the Measurement of 2S-2P Transition Frequencies of Muonic Hydrogen". Science. 339 (7303): 417–420. doi:10.1126/science.1230016. Retrieved 2013-01-25.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Headrick, J.M. (2005). "Spectral Signatures of Hydrated Proton Vibrations in Water Clusters". Science. 308 (5729): 1765–69. Bibcode:2005Sci...308.1765H. doi:10.1126/science.1113094. PMID 15961665.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
R.H. Petrucci, W.S. Harwood, and F.G. Herring (2002). General Chemistry (8th ed.). p. 41.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ See meeting report and announcement
- ^ A. Romer, Amer. J. Phys. 65, 707 (1997) Proton or prouton? Rutherford and the depths of the atom
- ^ Rutherford reported acceptance by the British Association in a footnote to a 1921 paper by O. Masson in the Philosophical Magazine (O. Masson, Phil. Mag. 41, 281, 1921)
- ^ Pais, Inward Bound, first edition, Oxford Press, 1986, page 296. Pais reported that he believed the first science literature use of the word proton occurs in the article Nature, 106, 357, 1920.
- ^ a b "Apollo 11 Mission". Lunar and Planetary Institute. 2009. Retrieved 2009-06-12.
- ^ a b c "Space Travel and Cancer Linked? Stony Brook Researcher Secures NASA Grant to Study Effects of Space Radiation". Brookhaven National Laboratory. 12 December 2007. Retrieved 2009-06-12.
- ^ a b
B. Shukitt-Hale, A. Szprengiel, J. Pluhar, B.M. Rabin, and J.A. Joseph. "The effects of proton exposure on neurochemistry and behavior". Elsevier/COSPAR. Retrieved 2009-06-12.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ N.W. Green and A.R. Frederickson. "A Study of Spacecraft Charging due to Exposure to Interplanetary Protons" (PDF). Jet Propulsion Laboratory. Retrieved 2009-06-12.
- ^ H. Planel (2004). Space and life: an introduction to space biology and medicine. CRC Press. pp. 135–138. ISBN 0-415-31759-2.
- ^ G. Gabrielse (2006). "Antiproton mass measurements". International Journal of Mass Spectrometry. 251 (2–3): 273–280. Bibcode:2006IJMSp.251..273G. doi:10.1016/j.ijms.2006.02.013.
