Telbivudine
 | |
| Clinical data | |
|---|---|
| Trade names | Tyzeka, Sebivo |
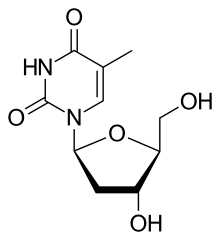
| Other names | 1-(2-deoxy-β-L-ribofuranosyl)-5-methyluracil β-L-2-deoxythymidine β-L-thymidine (LdT) 1-[(2S,4R,5S)-4-hydroxy-5-hydroxymethyltetrahydrofuran-2-yl]-5-methyl-1H-pyrimidine-2,4-dione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607045 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Low (3.3% in vitro) |
| Metabolism | Nil |
| Elimination half-life | 40 to 49 hours (terminal phase) |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.511 |
| Chemical and physical data | |
| Formula | C10H14N2O5 |
| Molar mass | 242.231 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Telbivudine is an antiviral drug used in the treatment of hepatitis B infection. It is marketed by Swiss pharmaceutical company Novartis under the trade names Sebivo (European Union) and Tyzeka (United States). Clinical trials have shown it to be significantly more effective than lamivudine or adefovir, and less likely to cause resistance.[1][2][3] However, HBV signature resistance mutation M204I (a change from methionine to isoleucine at position 204 in the reverse transcriptase domain of the hepatitis B polymerase) or L180M+M204V have been associated with Telbivudine resistance.[4]
Telbivudine is a synthetic thymidine β-L-nucleoside analogue; it is the L-isomer of thymidine. Telbivudine impairs hepatitis B virus (HBV) DNA replication by leading to chain termination. It differs from the natural nucleotide only with respect to the location of the sugar and base moieties, taking on an levorotatory configuration versus a dextrorotatory configuration as do the natural deoxynucleosides.[4] It is taken orally in a dose of 600 mg once daily with or without food.[5]
Telbivudine has no in vitro activity against HIV-1,[6] and in a case-series of three HIV-HBV co-infected patients, telbivudine did not produce sustained HIV-1 virologic suppression or induce any resistance mutations in HIV-1.[7]
Phase III clinical trials suggested that telbivudine put patients at greater risk for myopathy and peripheral neuropathy than the comparator drug lamivudine.[8] FDA required a required a risk evaluation and mitigation strategy (REMS) aiming to increase awareness of peripheral neuropathy by requiring distribution of a medication guide.[9]
In 2016, Novartis posted a discontinuation notice.[10][11] Efficacy or safety concerns were not cited as rationale for discontinuation, but rather "availability of alternative medications"; presumably this refers to tenofovir disoproxil, which became available as a generic medication in 2017, and is a safe and effective treatment for chronic HBV infection.
References
[edit]- ^ Lai CL, Leung N, Teo EK, Tong M, Wong F, Hann HW, et al. (August 2005). "A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B". Gastroenterology. 129 (2): 528–536. doi:10.1016/j.gastro.2005.05.053. PMID 16083710.
- ^ Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, et al. (December 2007). "Telbivudine versus lamivudine in patients with chronic hepatitis B". The New England Journal of Medicine. 357 (25): 2576–2588. doi:10.1056/NEJMoa066422. hdl:10722/57525. PMID 18094378.
- ^ Chan HL, Heathcote EJ, Marcellin P, Lai CL, Cho M, Moon YM, et al. (December 2007). "Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial". Annals of Internal Medicine. 147 (11): 745–754. doi:10.7326/0003-4819-147-11-200712040-00183. PMID 17909201. S2CID 24543064.
- ^ a b Osborn MK (2009). "Safety and efficacy of telbivudine for the treatment of chronic hepatitis B". Therapeutics and Clinical Risk Management. 5: 789–798. doi:10.2147/tcrm.s5318. PMC 2762437. PMID 19851526.
- ^ Drugs.com. "Tyzeka: Package Insert / Prescribing Information". Drugs.com. Retrieved 2023-06-01.
- ^ Lin K, Karwowska S, Lam E, Limoli K, Evans TG, Avila C (June 2010). "Telbivudine exhibits no inhibitory activity against HIV-1 clinical isolates in vitro". Antimicrobial Agents and Chemotherapy. 54 (6): 2670–2673. doi:10.1128/AAC.01703-09. PMC 2876362. PMID 20308377.
- ^ Milazzo L, Caramma I, Lai A, Violin M, De Maddalena C, Cesari M, et al. (2009). "Telbivudine in the treatment of chronic hepatitis B: experience in HIV type-1-infected patients naive for antiretroviral therapy". Antiviral Therapy. 14 (6): 869–872. doi:10.3851/IMP1303. PMID 19812451. S2CID 26840376.
- ^ Drugs.com. "Tyzeka: Package Insert / Prescribing Information". Drugs.com. Retrieved 2023-06-01.
- ^ FDA. "Risk Evaluation and Mitigation Strategy" (PDF). Retrieved 2023-06-01.
- ^ "HBV drug Tyzeka discontinued". www.healio.com. Retrieved 2021-01-11.
- ^ "FDA: Hepatitis B Drug Discontinued". MPR. 2016-10-05. Retrieved 2021-01-11.
External links
[edit]- "Telbivudine". Drug Information Portal. U.S. National Library of Medicine.
