Talk:Selegiline/Archive 1
| This is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 1 |
ninja vandalism
I wish the good doctor would notify people as to why contributions are removed from articles from non-bias and medical sources with references. I am getting a little bit tired of wasting my time attempting to contribute to the content of articles and having it wiped out almost as soon as it is added. I think this is unfair and unnecessary. Pogue 11:45, August 7, 2005 (UTC)
- You misunderstand the purpose of external links. It is not a replacement for information that could be included in the article. External links should be used for examples (e.g. of information already mentioned in the article) or as references (to support claims made in the article). Your links add none of that. JFW | T@lk 14:01, 7 August 2005 (UTC)
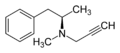
incorrect structure image
It's a carbon short - the group attached to the nitrogen should be a propargyl. —The preceding unsigned comment was added by Rndm (talk • contribs) .
- Oh crap. It is incorrect. I'll upload an accurate image tomorrow. --Bk0 (Talk) 06:16, 27 January 2006 (UTC)
- The image is now correct (no time like the present). Thank you for catching that mistake. --Bk0 (Talk) 06:39, 27 January 2006 (UTC)
image
Let's adopt for all chemical structures the official ChemIDPlus images and avoid changes. Jclerman 16:46, 29 October 2006 (UTC)
- That's a rather poor quality image. What exactly is "official" about ChemIDPlus? --Bk0 (Talk) 17:17, 29 October 2006 (UTC)
- "Official" is my abbreviation for the federal and private databases to which all numbers in the caption refer: Identifiers, i.e., CAS number 14611-51-9 [14611-52-0] (HCl), [2079-54-1] (deprenyl.HCl), [4530-70-5] ((+-)-isomer), [1205-70-5] ((+-)-isomer, HCl), [2323-36-6] (cpd w/o isomeric designation; deprenyl), [4528-51-2] ((S)-isomer), [4528-52-3] ((S)-isomer, HCl) ATC code N04BD01 PubChem 26757 DrugBank APRD00525 Chemical data Formula C13H17N
- The image is of poor quality because I can't generate a better one from the BMP file given by ChemIdPlus. Feel free to get a better image. Feel free to retrieve also the 3-D images and include them if you think they are useful. Jclerman 18:21, 29 October 2006 (UTC)
Links to some of the USA federal databases:
- What's wrong with the image I just added? Why replace it with the poor quality image from ChemIDPlus? I think mine's better - it's fully skeletal and hi-resolution.
Let's adopt for all chemical structures the CAS-ChemID-NLM standard images and avoid changes. Jclerman 16:46, 29 October 2006 (UTC)
- The image is of poor quality because I can't generate a better one from the BMP file given by ChemIdPlus. Feel free to generate a better image. Feel free to retrieve also the 3-D images and include them if you think they are useful. The links are shown above. IMHO the standard database image is more appropriate. Jclerman 18:21, 29 October 2006 (UTC)
- I don't agree that we should adopt images from a federal database when user-contributed images are higher quality and (possibly) more accurate/higher resolution. As long as the images are available under a compatible free license the best one should be used (which may or may not be a ChemIDPlus image). --Bk0 (Talk) 21:10, 29 October 2006 (UTC)
- Jclerman: why do you think the standard database image is more appropriate? I don't think it is. Please explain your reasoning. I'm going to leave your image where it is because I don't want to get into a revert war. You may have a very valid reason for preferring the ChemIdPlus image, but it would be better to discuss the matter and for all of us (plus, perhaps, the Wikiproject on Chemistry) to agree on a course of action. You're taking a rather unilateral approach, which tends not to suit Wikipedia.
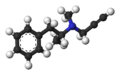
Here's a gallery of the images we have to choose from, with comments:
-
A: Fully skeletal structure.
-
B: ChemIDPlus image. JPEG formatting makes image look scruffy when resized. Low-resolution. Partially skeletal.
-
C: Fully skeletal structure, this one in the same configuration as the ChemIDPlus image.
-
D: User-made version of the ChemIDPlus image. Partially skeletal.
-
E: 3D ball-and-stick model.
-
F: Partially skeletal structure. One alkyne carbon is shown with a non-180° bond angle, which is incorrect.
Personally, I prefer fully skeletal images, because they are elegant and uncluttered. I think abelling terminal carbons and hydrogens is redundant - if you understand skeletal notation, you don't need them. If you don't understand skeletal notation, you won't understand the rest of the structure, so those carbons and hydrogens which are labelled don't help you very much
I think other users of this article will appreciate the lack of clutter in the fully skeletal images, which is why I am championing them. Fully skeletal structures are also standard in most chemistry, biology and medicine textbooks beyond high school level, and selegiline is a compound that is more likely to be discussed in advanced studies. They are also standard in scientific journals and reference works.
Ben 23:41, 29 October 2006 (UTC)
- I'm fine with fully skeletal images, and I personally feel the first image in the gallery above is preferable however it should be edited to show stereochemistry. Pharmaceutical selegiline is not racemic as far as I understand. The image from ChemIDPlus is poor quality and the other images are either unnecessarily contorted or inaccurate. --Bk0 (Talk) 00:48, 30 October 2006 (UTC)
- The first image does show stereochemistry - click on it to see.
- Ben 02:09, 30 October 2006 (UTC)
- I think that one must be internally consistent. The article's chembox has a link to an image in PubChem which is not the same as the one in ChemId. The one in PubChem should be consistent with the one in CAS which is not online. This is the one linked to the compound in the chembox:
- Interesting theme and nice to see a new player in Jclerman. "Let's adopt for all chemical structures " seems to beg for discussion at Wikiproject on Chemistry. Other comments - some of above arguments seem US-centric and pharma-oriented. Also, it seems that we've been doing pretty well image-wise, but I am willing to be educated.--Smokefoot 04:57, 30 October 2006 (UTC)
The .jpg image that is currently in the article (B) is clearly inferior to the other ones in the gallery above. There is no reason not to replace it. The images that Ben has created fit the guidelines set out by the Wikipedia:WikiProject Chemistry and are used probably hundreds of chemistry articles. My preference is A, but C would be fine to if it's desirable to keep consistency among phenethylamine articles. The image at Emsam should be replaced similarly as well. 148.177.1.213 12:35, 30 October 2006 (UTC)
pharmaceutical selegiline
Correct. It's no racemic: "ELDEPRYL (selegiline hydrochloride) is a levorotatory acetylenic derivative of phenethylamine. It is commonly referred to in the clinical and pharmacological literature as l-deprenyl." [From the manufacturer's info.]
Jclerman 03:14, 30 October 2006 (UTC)
- L-deprenyl is (obviously) not racemic, and is what the brands selegiline and eldepryl contain. Speaking of which, why is this page not called L-deprenyl? Anyway, a racemic mixture would mostly be useful in the treatment of ADHD, as the d-isomer is a potent and highly lipophilic dopamine reuptake inhibitor that metabolizes to d-methamphetamine, IIRC. Zuiram 16:50, 6 February 2007 (UTC)
deprenyl is published as making several species live longer Shattering the Barriers of Maximum Life Span: An Interview with Dr. Joseph Knoll
- This truly remarkable drug has also been shown to increase the maximum lifespan of laboratory animals by close to forty percent.
1-amphetamine?
Should this be L-amphetamine (and L-meth.) instead of 1- ? I think the referenced web site has the error in the abstract, probably mistook a lowercase l for a 1. I don't have access to the article to verify and I'm not sure so I won't correct it. Moo (talk) 10:33, 23 November 2007 (UTC)
trivia
I know that Trivia sections are generally frowned upon these days (and this is really only mildly amusing), so I thought I'd mention it here-- this drug is mentioned (as Deprenyl) in an episode of the X-Files where there is suspected poltergeist activity at a managed care acility (né nursing home). The drug is made to seem very dangerous and the conclusion of the episode mentions that trials of it were stopped after the incidents, yet here we are fourteen years later and it's already being used to pretty good effect. The times they are a changing, eh? 70.167.145.77 (talk) 05:10, 1 August 2008 (UTC)
Metabolite or not metabolite?
The metabolite of a drug, if known to be effective, is quickly siezed upon as an answer for its effectiveness. Codeine for instance is 10% metabolised to morphine, and is approximately 10% w/w as potent( beneath its ceiling dose) However, codeine`s oral activity/ availability is due to its -CH3 group not being rapidly metabolised on first-pass. İts activity is rapid, and binds well to opioid receptors. Therefore why should one not simply assume codeine is ~10% as active as morphine, is slowly metabolised and its metabolite may play a small role. Thus, selegiline a sympathomimetic, has properties akin to that group of medicines. İts metabolites may indeed play a small role in it`s activity, but it is the selegiline molecule that most probably does the work. İt is not a pro-drug. Profmad (talk) 17:53, 19 November 2008 (UTC)Profmad
Transdermal Bioavailability Questions
I have found two different sites with two different values for the bioavailability of the EMSAM patch, both of which differ from the number given on the page. http://jcp.sagepub.com/cgi/content/abstract/47/10/1256 Gives "an absolute bioavailability of 73%" while http://www.pbm.va.gov/Clinical%20Guidance/Drug%20Monographs/Selegiline%20Transdermal%20System%20(EMSAM).pdf Gives a bioavailability of 25-30%.
Now the second site seems more accurate in that it gives the actual amounts contained in EMSAM patches as 20,30,40mg, and 30% of those values gives the given value of 6/9/12 stated on the patches themselves. 74.74.185.45 (talk) 01:19, 3 June 2010 (UTC)
life extention
The article overlooks selegiline's use by life extentionists. It is one of the most promising substances studied for longevity. This link (one of many studies) may be useful Deprenyl (selegiline): Extending Life Span HansNZL (talk) 02:06, 31 July 2011 (UTC)
regulation
It says in the article that selegiline is an uncontrolled substance requiring a prescription. There are other, similarally unenlightened statements on other sites, so I wasn't able to find the clarification I need so that I understand. To my knowledge:
- The US controlled substances act is used to regulate drugs
- Schedule I of that act lists drugs and plants that are not to be made available except for research.
- Lower schedules, except the lowest, list drugs that are dangerous enough to require a prescription.
- The lowest schedule I thought, lists drugs that do not require a prescription. By tradition, many pharmacies may still opt not to sell these drugs without prescription.
If selegiline in the lower schedules? The lowest one? Is it unscheduled, but pharmacies still follow a tradition of only selling it by prescription? Or is there another law being enforced that requires it only be sold by prescription? If so, what law is this? --— robbie page talk 22:05, 24 July 2011 (UTC)
In the United States, the FDA determines what drugs require a prescription. If the DEA determines that it has jurisdiction over a potential drug of abuse, it separately sets guidelines that may impose similar restrictions. Penicillin would be another example of a drug restricted under one process but not another. See Prescription medication and associated links for further information. NScrutable (talk) 23:19, 25 October 2011 (UTC)
Vital Changes
This entire page is extremely inaccurate. No one here seems to know that Selegiline and Selegiline Hydrochloride (Hcl. - Eldepryl, Zelapar, all other Deprenyl products) are COMPLTELY different chemical entities. They should NOT be placed on the same page. Selegiline is a freebase liquid always in a natural state, and Selegiline Hydrochloride is NOT. They are not the same and produce different effects. Trials show this, especially one conducted by the New England Journal of Medicine.
If anyone is knowledgeable in the above regard, please comment below with suggested changes. This page needs extreme attention and is horribly inaccurate. Not many are knowledgeable at ALL with Selegiline and Selegiline Hcl, even pharmacists and pharmacies refer to the 2 as the same, or have "Selegiline, the generic for Eldepryl" in their computer system. HOWEVER, on all drug labels by whatever pharmaceutical company it explicitly calls the product SELEGILINE HCL.
Selegiline was produced a very long time ago, and has since been produced by only one pharmaceutical company. Discovery Experimental & Development Inc. (DEDI - now shut down) produced 99.99% pure Selegiline (ranging in purity 99.9923 - 99.9926), brand name Liquid Deprenyl Citrate. This article needs to really be separated into 2 articles or if so many are against it, it needs to be nearly 100% changed and re-written. Anyone learned in the issues above as I am, please reply to this, we will need to work together to put this article in a correct format which accurately depicts Selegiline. I'd like to gather ideas over the week, then make the changes. Jason1170 (talk) 07:27, 20 August 2008 (UTC)
- Your assertion that selegiline and selegiline HCl behave wildly differently seems unlikely, so please provide a reference - which NEJM trial are you referring to?
- Wikipedia almost never has separate articles for medicines that exist as freebases and as hydrochlorides or other salts. Probably because once the drug in question has dissolved, it is exactly the same molecule, regardless of whether it came from a freebase or salt. In this case, there would be an equilibrium: selegiline ⇌ [selegiline-H]+. The equilibrium position would depend on the pKaH of selegiline (which I don't know but would be similar to other amines, i.e. around 9).
- As for Liquid Deprenyl Citrate, the name indicates that it is selegiline citrate, not freebase selegiline at all. A quick Google search turns up nothing but fringe websites, such as http://www.liquid-deprenyl.com/. I couldn't find much mention of the citrate on Google Scholar (2 hits for the citrate vs. 577 for the hydrochloride).
- I agree with Ben. Different formulations, such as different salt forms, can have an effect on bioavailability and absorption, but they do not affect the inherent biological activity. The active chemical entity is the same once absorbed by the body. Having separate articles for different salt forms is not at all necessary. -- Ed (Edgar181) 14:15, 20 August 2008 (UTC)
- It does not matter for pharmacological activity if you have the freebase or any of the mentioned salts. The activity resides in the selegeline structure, not in the counter ion. Different salts are merely used to make the compound easier to handle and process or to increase stability. The freebase form can be used for faster absorption if it is not made into an oral drug. That mentioned website contains obvious quackery and nonsense. Cacycle (talk) 12:51, 27 August 2008 (UTC)
I agree with Jason. Disparaging DEDI as a fringe site is improper. The vast superiority of Liquid Deprenyl Citrate should be explored. I have seen its effects first-hand and they are remarkable. dave —Preceding unsigned comment added by 75.69.67.170 (talk) 22:58, 21 January 2011 (UTC)
- Doesn't change the fact that the chemical is essentially the same whether in freebase or salt form. — Preceding unsigned comment added by 196.30.79.194 (talk) 07:43, 11 June 2013 (UTC)
l-methamphetamine
As of several months ago, the article stated that the selegiline metabolite l-methamphetamine is inactive, meaning that it is minimally psychoactive. Recently the article was edited (I believe by Jclerman) to the current text: "Selegiline is partly metabolized to l-methamphetamine, an active stereoisomer of methamphetamine in vivo".
I believe this is incorrect, as the methamphetamine article states: "L-methamphetamine (also called levmetamfetamine and desoxyephedrine) has nasal decongestant activity and no abuse potential. This is the active ingredient in the US Vicks Inhaler." Also, l-methamphetamine is not produced illicitly for abuse purposes (the most common precursors used result in d-methamphetamine). For this reason I will edit this information to make it clear that l-methamphetamine has little or no abuse potential and the use of selegiline can not result in a methamphetamine-like intoxication. The use of the term 'active' and 'inactive' is admittedly vague and will be changed. --Bk0 (Talk) 18:38, 30 October 2006 (UTC)
- L-methamphetamine is orally active, etc., but you need a four times as high dose (compared to d-methamphetamine) to get the same level of CNS stimulation, meaning you need several 10mg tablets of selegiline to get the same effect as a single 5mg tablet of desoxyn (or even dexedrine).
- Also, l-meth is more peripherally active than d-meth.
- It is quite possible to get the CNS stimulant effect from it, particularly since the MAOI activity will potentiate it, but it requires a fairly high dose, and you'd have to be pretty desperate. There's a million other things one can abuse. As readily available as meth is in Japan, I fail to see why they'd latch onto something like l-dep... Zuiram 17:12, 6 February 2007 (UTC)
- Do you have any references for that? I've never heard of anyone successfully abusing selegiline for meth-like effects. In fact, combining sympathomimetic stimulants and MAOIs (which high-dose selegiline would be) is extraordinarily dangerous, often resulting in hypertensive crises. --Bk0 (Talk) 00:28, 7 February 2007 (UTC)
- Not if the selegiline dose is low enough to be selective. — Preceding unsigned comment added by 196.30.79.194 (talk) 06:34, 11 June 2013 (UTC)
- Do you have any references for that? I've never heard of anyone successfully abusing selegiline for meth-like effects. In fact, combining sympathomimetic stimulants and MAOIs (which high-dose selegiline would be) is extraordinarily dangerous, often resulting in hypertensive crises. --Bk0 (Talk) 00:28, 7 February 2007 (UTC)
L-methamphetamine is VERY abusable.... just google some info on inhaler abuse, or break one and consume the medicated "cotton" inside to see for yourself. Btw, same excuse was used for classical amphetamine and D-amphetamine 40-50 years ago, when people were abusing the "cottons" from Dexedrine inhalers left and right. Going back further yet, Bayer popularized a new OTC painkiller/antidiarrheal/antitussive branded "Heroin" as a non-addictive alternative to morphine. Hmm. — Preceding unsigned comment added by 208.127.93.10 (talk) 05:21, 8 November 2014 (UTC)
construction
sorry for the delay, i am looking for sources on who first this brought to market in Europe for PD... Jytdog (talk) 19:12, 5 February 2016 (UTC)
- note - I have not been able to find good sources yet on how selegiline was used in the clinic (and sold by Chinoin, I guess...) in Europe after it was discovered there. Some of the sources hinted that it was clinically used right away as an anti-depressant (things used to go pretty fast from discovery and publication to clinical use) but that fell into disuse along with the other MAOis.... and the PD use came along later. I am not sure who Somerset acquired the US rights from in the 1980s. hm. Maybe Chinoin but am not sure. Jytdog (talk) 20:39, 5 February 2016 (UTC)
External links modified
Hello fellow Wikipedians,
I have just modified one external link on Selegiline. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20101129015812/http://www.lloydinc.com/pdfs/Endocrinology/Vol14_issue3_2004.pdf to http://www.lloydinc.com/pdfs/Endocrinology/Vol14_issue3_2004.pdf
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 05:02, 26 December 2017 (UTC)
selectivity
Actually, already at 10mg/day, the MAO-B inhibition is 90% or better, and a handful of people have reported hypertensive reactions at 10mg/day, so it's not unlikely that there is a sliding transition from full selectivity (<10mg/day) to no selectivity (>60mg/day). As a nonselective MAOI, it is administered at doses from 20mg/day and up, with 20mg-60mg being the effective range.
- Thank you for your suggestion! When you feel an article needs improvement, please feel free to make whatever changes you feel are needed. Wikipedia is a wiki, so anyone can edit almost any article by simply following the Edit this page link at the top. You don't even need to log in! (Although there are some reasons why you might like to…) The Wikipedia community encourages you to be bold. Don't worry too much about making honest mistakes—they're likely to be found and corrected quickly. If you're not sure how editing works, check out how to edit a page, or use the sandbox to try out your editing skills. New contributors are always welcome. JFW | T@lk 12:04, 8 Jun 2005 (UTC)