Threonine
| |

| |
| Names | |
|---|---|
| IUPAC name
Threonine
| |
| Other names
2-Amino-3-hydroxybutanoic acid
| |
| Identifiers | |
| ECHA InfoCard | 100.000.704 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C4H9NO3 | |
| Molar mass | 119.120 g·mol−1 |
| Supplementary data page | |
| Threonine (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Threonine (abbreviated as Thr or T)[1] is an α-amino acid with the chemical formula HO2CCH(NH2)CH(OH)CH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar. Together with serine, threonine is one of two proteinogenic amino acids bearing an alcohol group (tyrosine is not an alcohol but a phenol since its hydroxyl group is bonded directly to an aromatic ring, giving it different acid/base and oxidative properties).
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxy side chain can undergo O-linked glycosylation. In addition, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine.
History
Threonine was discovered as the last of the 20 common proteinogenic amino acids in the 1930s by William Cumming Rose.
Stereoisomerism
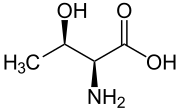
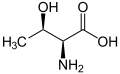  |
| L-Threonine (2S,3R) and D-Threonine (2R,3S) |
 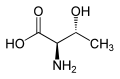 |
| L-allo-Threonine (2S,3S) and D-allo-Threonine (2R,3R) |
With two chiral centers, threonine can exist in four possible stereoisomers with the following configurations: (2S,3R), (2R,3S), (2S,3S) and (2R,3R). However, the name L-threonine is used for one single enantiomer, (2S,3R)-2-amino-3-hydroxybutanoic acid. The second stereoisomer (2S,3S), which is rarely present in nature, is called L-allo-threonine. The two stereoisomers (2R,3S)- and (2R,3R)-2-amino-3-hydroxybutanoic acid are only of minor importance.
Biosynthesis
As an essential amino acid, threonine is not synthesized in humans, hence we must ingest threonine in the form of threonine-containing proteins. In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O-phosphorylation; this phosphate ester undergoes hydrolysis concomitant with relocation of the OH group.[2] Enzymes involved in a typical biosynthesis of threonine include:
- aspartokinase
- α-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine kinase
- threonine synthase.

Metabolism
Threonine is metabolized in two ways:
- It is converted to pyruvate via threonine dehydrogenase. An intermediate in this pathway can undergo thiolysis with CoA to produce acetyl-CoA and glycine.
- In humans, it is converted to alpha-ketobutyrate in a less common pathway via the enzyme serine dehydratase, and thereby enters the pathway leading to succinyl-CoA.
Sources
Foods high in threonine include cottage cheese, poultry, fish, meat, lentils, and sesame seeds.
Racemic threonine can be prepared from crotonic acid by alpha-functionalization using mercury(II) acetate.[3]
References
- ^ "Nomenclature and symbolism for amino acids and peptides (IUPAC-IUB Recommendations 1983)", Pure Appl. Chem., 56 (5): 595–624, 1984, doi:10.1351/pac198456050595.
- ^ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000). Principles of Biochemistry (3rd ed.). New York: W. H. Freeman. ISBN 1-57259-153-6..
- ^ Carter, Herbert E.; West, Harold D. (1940). "dl-Threonine". Organic Syntheses. 20: 101; Collected Volumes, vol. 3, p. 813..
External links

