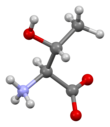
Threonine
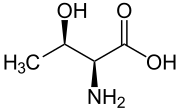 Skeletal formula of L-threonine
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Threonine
| |||
| Other names
2-Amino-3-hydroxybutanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| DrugBank |
| ||
| ECHA InfoCard | 100.000.704 | ||
| EC Number |
| ||
| |||
| KEGG |
| ||
PubChem CID
|
| ||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H9NO3 | |||
| Molar mass | 119.120 g·mol−1 | ||
| (H2O, g/dl) 10.6(30°),14.1(52°),19.0(61°) | |||
| Acidity (pKa) | 2.63 (carboxyl), 10.43 (amino)[1] | ||
| Supplementary data page | |||
| Threonine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Threonine (symbol Thr or T)[2] is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− form when dissolved in water), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as E. coli.[3] It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG).
Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices).
Modifications[edit]
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can undergo O-linked glycosylation. In addition, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine. Phosphothreonine has three potential coordination sites (carboxyl, amine and phosphate group) and determination of the mode of coordination between phosphorylated ligands and metal ions occurring in an organism is important to explain the function of the phosphothreonine in biological processes.[4]
History[edit]
Threonine was the last of the 20 common proteinogenic amino acids to be discovered. It was discovered in 1936 by William Cumming Rose,[5] collaborating with Curtis Meyer. The amino acid was named threonine because it was similar in structure to threonic acid, a four-carbon monosaccharide with molecular formula C4H8O5[6]
Stereoisomers[edit]
  |
| L-threonine (2S,3R) and D-threonine (2R,3S) |
  |
| L-allothreonine (2S,3S) and D-allothreonine (2R,3R) |
Threonine is one of two proteinogenic amino acids with two stereogenic centers, the other being isoleucine. Threonine can exist in four possible stereoisomers with the following configurations: (2S,3R), (2R,3S), (2S,3S) and (2R,3R). However, the name L-threonine is used for one single stereoisomer, (2S,3R)-2-amino-3-hydroxybutanoic acid. The stereoisomer (2S,3S), which is rarely present in nature, is called L-allothreonine.[7]
Biosynthesis[edit]
As an essential amino acid, threonine is not synthesized in humans, and needs to be present in proteins in the diet. Adult humans require about 20 mg/kg body weight/day.[8] In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O-phosphorylation; this phosphate ester undergoes hydrolysis concomitant with relocation of the OH group.[9] Enzymes involved in a typical biosynthesis of threonine include:
- aspartokinase
- β-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine kinase
- threonine synthase.

Metabolism[edit]
Threonine is metabolized in at least three ways:
- In many animals it is converted to pyruvate via threonine dehydrogenase. An intermediate in this pathway can undergo thiolysis with CoA to produce acetyl-CoA and glycine.
- In humans the gene for threonine dehydrogenase is an inactive pseudogene,[10] so threonine is converted to α-ketobutyrate. The mechanism of the first step is analogous to that catalyzed by serine dehydratase, and the serine and threonine dehydratase reactions are probably catalyzed by the same enzyme.[11]
- In many organisms it is O-phosphorylated by a kinase preparatory to further metabolism. This is especially important in bacteria as part of the biosynthesis of cobalamin (Vitamin B12), as the product is converted to (R)-1-aminopropan-2-ol for incorporation into the vitamin's sidechain.[12]
- Threonine is used to synthesize glycine during the endogenous production of L-carnitine in the brain and liver of rats.[13][14]
Metabolic diseases[edit]
The degradation of threonine is impaired in the following metabolic diseases:
- Combined malonic and methylmalonic aciduria (CMAMMA)[15]
- Methylmalonic acidemia[15]
- Propionic acidemia[16]
Research of Threonine as a Dietary Supplement in Animals[edit]
Effects of threonine dietary supplementation have been researched in broilers.[17]
An essential amino acid, threonine is involved in the metabolism of fats, the creation of proteins, the proliferation and differentiation of embryonic stem cells, and the health and function of the intestines. Animal health and illness are strongly correlated with the need for and metabolism of threonine. Intestinal inflammation and energy metabolism disorders in animals may be alleviated by appropriate amounts of dietary threonine. Nevertheless, because these effects pertain to the control of nutrition metabolism, more research is required to confirm the results in various animal models. Furthermore, more research is needed to understand how threonine controls the dynamic equilibrium of the intestinal barrier function, immunological response and gut flora.[18]
Sources[edit]
Foods high in threonine include cottage cheese, poultry, fish, meat, lentils, black turtle bean[19] and sesame seeds.[20]
Racemic threonine can be prepared from crotonic acid by alpha-functionalization using mercury(II) acetate.[21]
References[edit]
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- ^ Raïs, Badr; Chassagnole, Christophe; Lettelier, Thierry; Fell, David; Mazat, Jean-Pierre (2001). "Threonine synthesis from aspartate in Escherichia coli cell-free extracts: pathway dynamics". Biochem J. 356 (Pt 2): 425–32. doi:10.1042/bj3560425. PMC 1221853. PMID 11368769.
- ^ Jastrzab, Renata (2013). "Studies of new phosphothreonine complexes formed in binary and ternary systems including biogenic amines and copper(II)". Journal of Coordination Chemistry. 66 (1): 98–113. doi:10.1080/00958972.2012.746678
- ^ A Dictionary of scientists. Daintith, John., Gjertsen, Derek. Oxford: Oxford University Press. 1999. p. 459. ISBN 9780192800862. OCLC 44963215.
{{cite book}}: CS1 maint: others (link) - ^ Meyer, Curtis (20 July 1936). "The Spatial Configuation of Alpha-Amino-Beta-Hydroxy-n-Butyric Acid" (PDF). Journal of Biological Chemistry. 115 (3): 721–729. doi:10.1016/S0021-9258(18)74711-X.
- ^ "Nomenclature and symbolism for amino acids and peptides (Recommendations 1983)". Pure and Applied Chemistry. 56 (5): 601, 603, 608. 1 January 1984. doi:10.1351/pac198456050595.
- ^ Institute of Medicine (2002). "Protein and Amino Acids". Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. pp. 589–768. doi:10.17226/10490. ISBN 978-0-309-08525-0.
- ^ Lehninger, Albert L.; Nelson, David L.; Cox, Michael M. (2000). Principles of Biochemistry (3rd ed.). New York: W. H. Freeman. ISBN 1-57259-153-6..
- ^ Stipanuk, Martha H.; Caudill, Marie A. (2013). Biochemical, Physiological, and Molecular Aspects of Human Nutrition – E-Book. Elsevier Health Sciences. ISBN 9780323266956.
- ^ Bhardwaj, Uma; Bhardwaj, Ravindra. Biochemistry for Nurses. Pearson Education India. ISBN 9788131795286.
- ^ Fang, H; Kang, J; Zhang, D (30 January 2017). "Microbial production of vitamin B12: a review and future perspectives". Microbial Cell Factories. 16 (1): 15. doi:10.1186/s12934-017-0631-y. PMC 5282855. PMID 28137297.
- ^ Adeva-Andany, M; Souto-Adeva, G; Ameneiros-Rodríguez, E; Fernández-Fernández, C; Donapetry-García, C; Domínguez-Montero, A (January 2018). "Insulin resistance and glycine metabolism in humans". Amino Acids. 50 (1): 11–27. doi:10.1007/s00726-017-2508-0. PMID 29094215. S2CID 3708658.
- ^ Dalangin, R; Kim, A; Campbell, RE (27 August 2020). "The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection". International Journal of Molecular Sciences. 21 (17): 6197. doi:10.3390/ijms21176197. PMC 7503967. PMID 32867295.
- ^ a b Manoli, Irini; Sloan, Jennifer L.; Venditti, Charles P. (1993), Adam, Margaret P.; Feldman, Jerry; Mirzaa, Ghayda M.; Pagon, Roberta A. (eds.), "Isolated Methylmalonic Acidemia", GeneReviews®, Seattle (WA): University of Washington, Seattle, PMID 20301409, retrieved 2024-03-09
- ^ Shchelochkov, Oleg A.; Carrillo, Nuria; Venditti, Charles (1993), Adam, Margaret P.; Feldman, Jerry; Mirzaa, Ghayda M.; Pagon, Roberta A. (eds.), "Propionic Acidemia", GeneReviews®, Seattle (WA): University of Washington, Seattle, PMID 22593918, retrieved 2024-03-09
- ^ Qaisrani, Shafqat Nawaz; Ahmed, Ibrar; Azam, Faheem; Bibi, Fehmida; Saima; Pasha, Talat Naseer; Azam, Farooq (2018-07-01). "Threonine in broiler diets: an updated review". Annals of Animal Science. 18 (3): 659–674. doi:10.2478/aoas-2018-0020. ISSN 2300-8733.
- ^ Tang, Qi; Peng, Tan; Ning, Ma; Xi, Ma (2021-07-28). "Physiological Functions of Threonine in Animals: Beyond Nutrition Metabolism". Nutrients. 13 (8): 2592. doi:10.3390/nu13082592. PMC 8399342. PMID 34444752.
- ^ "Error". ndb.nal.usda.gov. Archived from the original on 2018-11-16. Retrieved 2013-05-29.
- ^ "SELF Nutrition Data - Food Facts, Information & Calorie Calculator". nutritiondata.self.com. Retrieved 27 March 2018.
- ^ Carter, Herbert E.; West, Harold D. (1940). "dl-Threonine". Organic Syntheses. 20: 101; Collected Volumes, vol. 3, p. 813..