Type 2 diabetes: Difference between revisions
→Diagnosis: Substituted template per Wikipedia:Templates for discussion/Log/2022 November 16#Template:OGTT2. |
→Prevention: Updated section using conclusions of Cambpell Collaboration Systematic Review Madsen, K. S., Chi, Y., Metzendorf, M. I., Richter, B., & Hemmingsen, B. (2019). Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. In Cochrane Database of Systematic Reviews (Vol. 2019, Issue 12). John Wiley and Sons Ltd. https://doi.org/10.1002/14651858.CD008558.pub2 |
||
| Line 134: | Line 134: | ||
Onset of type 2 diabetes can be delayed or prevented through proper nutrition and regular exercise.<ref>{{cite journal | vauthors = Raina Elley C, Kenealy T | title = Lifestyle interventions reduced the long-term risk of diabetes in adults with impaired glucose tolerance | journal = Evidence-Based Medicine | volume = 13 | issue = 6 | pages = 173 | date = December 2008 | pmid = 19043031 | doi = 10.1136/ebm.13.6.173 | s2cid = 26714233 }}</ref><ref>{{cite journal | vauthors = Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I, Figuls M, Metzendorf MI, Richter B | title = Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus | journal = The Cochrane Database of Systematic Reviews | volume = 2017 | issue = 12 | pages = CD003054 | date = December 2017 | pmid = 29205264 | pmc = 6486271 | doi = 10.1002/14651858.CD003054.pub4 }}</ref> Intensive lifestyle measures may reduce the risk by over half.<ref name=AFP09/><ref name=Schellenberg2013>{{cite journal | vauthors = Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C | title = Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis | journal = Annals of Internal Medicine | volume = 159 | issue = 8 | pages = 543–551 | date = October 2013 | pmid = 24126648 | doi = 10.7326/0003-4819-159-8-201310150-00007 | doi-access = free }}</ref> The benefit of exercise occurs regardless of the person's initial weight or subsequent weight loss.<ref>{{cite journal | vauthors = O'Gorman DJ, Krook A | title = Exercise and the treatment of diabetes and obesity | journal = The Medical Clinics of North America | volume = 95 | issue = 5 | pages = 953–969 | date = September 2011 | pmid = 21855702 | doi = 10.1016/j.mcna.2011.06.007 }}</ref> High levels of physical activity reduce the risk of diabetes by about 28%.<ref name=BMJ2016>{{cite journal | vauthors = Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, Cercy K, Vos T, Murray CJ, Forouzanfar MH | display-authors = 6 | title = Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013 | journal = BMJ | volume = 354 | pages = i3857 | date = August 2016 | pmid = 27510511 | pmc = 4979358 | doi = 10.1136/bmj.i3857 | doi-access = free }}</ref> Evidence for the benefit of dietary changes alone, however, is limited,<ref>{{cite journal | vauthors = Nield L, Summerbell CD, Hooper L, Whittaker V, Moore H | title = Dietary advice for the prevention of type 2 diabetes mellitus in adults | journal = The Cochrane Database of Systematic Reviews | issue = 3 | pages = CD005102 | date = July 2008 | pmid = 18646120 | doi = 10.1002/14651858.CD005102.pub2 | veditors = Nield L | s2cid = 23039006 | hdl = 10149/92337 }}{{Retracted|intentional=yes}}</ref> with some evidence for a diet high in green leafy vegetables<ref>{{cite journal | vauthors = Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ | title = Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis | journal = BMJ | volume = 341 | pages = c4229 | date = August 2010 | pmid = 20724400 | pmc = 2924474 | doi = 10.1136/bmj.c4229 }}</ref> and some for limiting the intake of sugary drinks.<ref name="Schwing">{{cite journal | vauthors = Schwingshackl L, Hoffmann G, Lampousi AM, Knüppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H | display-authors = 6 | title = Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies | journal = European Journal of Epidemiology | volume = 32 | issue = 5 | pages = 363–375 | date = May 2017 | pmid = 28397016 | pmc = 5506108 | doi = 10.1007/s10654-017-0246-y }}</ref> There is an association between higher intake of sugar-sweetened fruit juice and diabetes, but no evidence of an association with 100% fruit juice.<ref>{{cite journal | vauthors = Xi B, Li S, Liu Z, Tian H, Yin X, Huai P, Tang W, Zhou D, Steffen LM | display-authors = 6 | title = Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis | journal = PLOS ONE | volume = 9 | issue = 3 | pages = e93471 | date = 2014 | pmid = 24682091 | pmc = 3969361 | doi = 10.1371/journal.pone.0093471 | doi-access = free | bibcode = 2014PLoSO...993471X }}</ref> A 2019 review found evidence of benefit from [[dietary fiber]].<ref>{{cite journal | vauthors = Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L | title = Carbohydrate quality and human health: a series of systematic reviews and meta-analyses | journal = Lancet | volume = 393 | issue = 10170 | pages = 434–445 | date = February 2019 | pmid = 30638909 | doi = 10.1016/S0140-6736(18)31809-9 | s2cid = 58632705 | doi-access = free }}</ref> |
Onset of type 2 diabetes can be delayed or prevented through proper nutrition and regular exercise.<ref>{{cite journal | vauthors = Raina Elley C, Kenealy T | title = Lifestyle interventions reduced the long-term risk of diabetes in adults with impaired glucose tolerance | journal = Evidence-Based Medicine | volume = 13 | issue = 6 | pages = 173 | date = December 2008 | pmid = 19043031 | doi = 10.1136/ebm.13.6.173 | s2cid = 26714233 }}</ref><ref>{{cite journal | vauthors = Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I, Figuls M, Metzendorf MI, Richter B | title = Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus | journal = The Cochrane Database of Systematic Reviews | volume = 2017 | issue = 12 | pages = CD003054 | date = December 2017 | pmid = 29205264 | pmc = 6486271 | doi = 10.1002/14651858.CD003054.pub4 }}</ref> Intensive lifestyle measures may reduce the risk by over half.<ref name=AFP09/><ref name=Schellenberg2013>{{cite journal | vauthors = Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C | title = Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis | journal = Annals of Internal Medicine | volume = 159 | issue = 8 | pages = 543–551 | date = October 2013 | pmid = 24126648 | doi = 10.7326/0003-4819-159-8-201310150-00007 | doi-access = free }}</ref> The benefit of exercise occurs regardless of the person's initial weight or subsequent weight loss.<ref>{{cite journal | vauthors = O'Gorman DJ, Krook A | title = Exercise and the treatment of diabetes and obesity | journal = The Medical Clinics of North America | volume = 95 | issue = 5 | pages = 953–969 | date = September 2011 | pmid = 21855702 | doi = 10.1016/j.mcna.2011.06.007 }}</ref> High levels of physical activity reduce the risk of diabetes by about 28%.<ref name=BMJ2016>{{cite journal | vauthors = Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, Cercy K, Vos T, Murray CJ, Forouzanfar MH | display-authors = 6 | title = Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013 | journal = BMJ | volume = 354 | pages = i3857 | date = August 2016 | pmid = 27510511 | pmc = 4979358 | doi = 10.1136/bmj.i3857 | doi-access = free }}</ref> Evidence for the benefit of dietary changes alone, however, is limited,<ref>{{cite journal | vauthors = Nield L, Summerbell CD, Hooper L, Whittaker V, Moore H | title = Dietary advice for the prevention of type 2 diabetes mellitus in adults | journal = The Cochrane Database of Systematic Reviews | issue = 3 | pages = CD005102 | date = July 2008 | pmid = 18646120 | doi = 10.1002/14651858.CD005102.pub2 | veditors = Nield L | s2cid = 23039006 | hdl = 10149/92337 }}{{Retracted|intentional=yes}}</ref> with some evidence for a diet high in green leafy vegetables<ref>{{cite journal | vauthors = Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ | title = Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis | journal = BMJ | volume = 341 | pages = c4229 | date = August 2010 | pmid = 20724400 | pmc = 2924474 | doi = 10.1136/bmj.c4229 }}</ref> and some for limiting the intake of sugary drinks.<ref name="Schwing">{{cite journal | vauthors = Schwingshackl L, Hoffmann G, Lampousi AM, Knüppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H | display-authors = 6 | title = Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies | journal = European Journal of Epidemiology | volume = 32 | issue = 5 | pages = 363–375 | date = May 2017 | pmid = 28397016 | pmc = 5506108 | doi = 10.1007/s10654-017-0246-y }}</ref> There is an association between higher intake of sugar-sweetened fruit juice and diabetes, but no evidence of an association with 100% fruit juice.<ref>{{cite journal | vauthors = Xi B, Li S, Liu Z, Tian H, Yin X, Huai P, Tang W, Zhou D, Steffen LM | display-authors = 6 | title = Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis | journal = PLOS ONE | volume = 9 | issue = 3 | pages = e93471 | date = 2014 | pmid = 24682091 | pmc = 3969361 | doi = 10.1371/journal.pone.0093471 | doi-access = free | bibcode = 2014PLoSO...993471X }}</ref> A 2019 review found evidence of benefit from [[dietary fiber]].<ref>{{cite journal | vauthors = Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L | title = Carbohydrate quality and human health: a series of systematic reviews and meta-analyses | journal = Lancet | volume = 393 | issue = 10170 | pages = 434–445 | date = February 2019 | pmid = 30638909 | doi = 10.1016/S0140-6736(18)31809-9 | s2cid = 58632705 | doi-access = free }}</ref> |
||
In those with [[impaired glucose tolerance]], a 2019 [[systematic review]] found moderate-quality evidence that [[Metformin]], when compared to diet and exercise or a [[placebo]] intervention, appeared to delay or reduce the risk of developing type 2 diabetes.<ref name=":3">{{Cite journal |last=Madsen |first=Kasper S |last2=Chi |first2=Yuan |last3=Metzendorf |first3=Maria-Inti |last4=Richter |first4=Bernd |last5=Hemmingsen |first5=Bianca |date=2019-12-03 |editor-last=Cochrane Metabolic and Endocrine Disorders Group |title=Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus |url=http://doi.wiley.com/10.1002/14651858.CD008558.pub2 |journal=Cochrane Database of Systematic Reviews |language=en |volume=2019 |issue=12 |doi=10.1002/14651858.CD008558.pub2 |pmc=PMC6889926 |pmid=31794067}}</ref> This same review found moderate-quality evidence that when compared to intensive diet and exercise, Metformin did not reduce risk of developing type 2 diabetes, as well as very low-quality evidence that combining Metformin with intensive diet and exercise does not appear to have any effect on risk of developing type 2 diabetes when compared to intensive diet and exercise alone.<ref name=":3" /> This systematic review only found one suitable trial comparing Metformin with [[Sulfonylurea|Sulphonylurea]] in reducing risk of type 2 diabetes but it did not report any patient-relevant outcomes.<ref name=":3" /> |
|||
| ⚫ | |||
| ⚫ | A 2017 review found that, long term, lifestyle changes decreased the risk by 28%, while medication does not reduce risk after withdrawal.<ref>{{cite journal | vauthors = Haw JS, Galaviz KI, Straus AN, Kowalski AJ, Magee MJ, Weber MB, Wei J, Narayan KM, Ali MK | title = Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials | journal = JAMA Internal Medicine | volume = 177 | issue = 12 | pages = 1808–1817 | date = December 2017 | pmid = 29114778 | pmc = 5820728 | doi = 10.1001/jamainternmed.2017.6040 }}</ref> While low [[vitamin D]] levels are associated with an increased risk of diabetes, correcting the levels by supplementing vitamin D3 does not improve that risk.<ref>{{cite journal | vauthors = Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA | title = Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis | journal = The Journal of Clinical Endocrinology and Metabolism | volume = 99 | issue = 10 | pages = 3551–60 | date = October 2014 | pmid = 25062463 | pmc = 4483466 | doi = 10.1210/jc.2014-2136 }}</ref> |
||
==Management== |
==Management== |
||
Revision as of 12:00, 26 November 2022
| Type 2 diabetes | |
|---|---|
| Other names | Diabetes mellitus type 2; adult-onset diabetes;[1] noninsulin-dependent diabetes mellitus (NIDDM) |
 | |
| A blue circle is the universal symbol of diabetes[2] | |
| Pronunciation | |
| Specialty | Endocrinology |
| Symptoms | Increased thirst, frequent urination, unexplained weight loss, increased hunger[3] |
| Complications | Hyperosmolar hyperglycemic state, diabetic ketoacidosis, heart disease, strokes, diabetic retinopathy, kidney failure, amputations[1][4][5] |
| Usual onset | Middle or older age[6] |
| Duration | Long term[6] |
| Causes | Obesity, lack of exercise, genetics[1][6] |
| Diagnostic method | Blood test[3] |
| Prevention | Maintaining normal weight, exercising, eating properly[1] |
| Treatment | Dietary changes, metformin, insulin, bariatric surgery[1][7][8][9] |
| Prognosis | 10 year shorter life expectancy[10] |
| Frequency | 392 million (2015)[11] |
Type 2 diabetes, formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin.[6] Common symptoms include increased thirst, frequent urination, and unexplained weight loss.[3] Symptoms may also include increased hunger, feeling tired, and sores that do not heal.[3] Often symptoms come on slowly.[6] Long-term complications from high blood sugar include heart disease, strokes, diabetic retinopathy which can result in blindness, kidney failure, and poor blood flow in the limbs which may lead to amputations.[1] The sudden onset of hyperosmolar hyperglycemic state may occur; however, ketoacidosis is uncommon.[4][5]
Type 2 diabetes primarily occurs as a result of obesity and lack of exercise.[1] Some people are genetically more at risk than others.[6]
Type 2 diabetes makes up about 90% of cases of diabetes, with the other 10% due primarily to type 1 diabetes and gestational diabetes.[1] In type 1 diabetes there is a lower total level of insulin to control blood glucose, due to an autoimmune induced loss of insulin-producing beta cells in the pancreas.[12][13] Diagnosis of diabetes is by blood tests such as fasting plasma glucose, oral glucose tolerance test, or glycated hemoglobin (A1C).[3]
Type 2 diabetes is largely preventable by staying a normal weight, exercising regularly, and eating a healthy diet (high in fruits and vegetables and low in sugar and saturated fats).[1] Treatment involves exercise and dietary changes.[1] If blood sugar levels are not adequately lowered, the medication metformin is typically recommended.[7][14] Many people may eventually also require insulin injections.[9] In those on insulin, routinely checking blood sugar levels is advised; however, this may not be needed in those taking pills.[15] Bariatric surgery often improves diabetes in those who are obese.[8][16]
Rates of type 2 diabetes have increased markedly since 1960 in parallel with obesity.[17] As of 2015 there were approximately 392 million people diagnosed with the disease compared to around 30 million in 1985.[11][18] Typically it begins in middle or older age,[6] although rates of type 2 diabetes are increasing in young people.[19][20] Type 2 diabetes is associated with a ten-year-shorter life expectancy.[10] Diabetes was one of the first diseases ever described, dating back to an Egyptian manuscript from c. 1500 BCE.[21] The importance of insulin in the disease was determined in the 1920s.[22]
Signs and symptoms

The classic symptoms of diabetes are frequent urination (polyuria), increased thirst (polydipsia), increased hunger (polyphagia), and weight loss.[23] Other symptoms that are commonly present at diagnosis include a history of blurred vision, itchiness, peripheral neuropathy, recurrent vaginal infections, and fatigue.[13] Other symptoms may include loss of taste.[24] Many people, however, have no symptoms during the first few years and are diagnosed on routine testing.[13] A small number of people with type 2 diabetes can develop a hyperosmolar hyperglycemic state (a condition of very high blood sugar associated with a decreased level of consciousness and low blood pressure).[13]
Complications
Type 2 diabetes is typically a chronic disease associated with a ten-year-shorter life expectancy.[10] This is partly due to a number of complications with which it is associated, including: two to four times the risk of cardiovascular disease, including ischemic heart disease and stroke; a 20-fold increase in lower limb amputations, and increased rates of hospitalizations.[10] In the developed world, and increasingly elsewhere, type 2 diabetes is the largest cause of nontraumatic blindness and kidney failure.[25] It has also been associated with an increased risk of cognitive dysfunction and dementia through disease processes such as Alzheimer's disease and vascular dementia.[26] Other complications include hyperpigmentation of skin (acanthosis nigricans), sexual dysfunction, and frequent infections.[23] There is also an association between type 2 diabetes and mild hearing loss.[27]
Causes
The development of type 2 diabetes is caused by a combination of lifestyle and genetic factors.[25][28] While some of these factors are under personal control, such as diet and obesity, other factors are not, such as increasing age, female sex, and genetics.[10] Obesity is more common in women than men in many parts of Africa.[29] The nutritional status of a mother during fetal development may also play a role, with one proposed mechanism being that of DNA methylation.[30] The intestinal bacteria Prevotella copri and Bacteroides vulgatus have been connected with type 2 diabetes.[31]
Lifestyle
Lifestyle factors are important to the development of type 2 diabetes, including obesity and being overweight (defined by a body mass index of greater than 25), lack of physical activity, poor diet, psychological stress, and urbanization.[10][32] Excess body fat is associated with 30% of cases in those of Chinese and Japanese descent, 60–80% of cases in those of European and African descent, and 100% of cases in Pima Indians and Pacific Islanders.[13] Among those who are not obese, a high waist–hip ratio is often present.[13] Smoking appears to increase the risk of type 2 diabetes.[33] A lack of sleep has also been linked to type 2 diabetes.[34] Laboratory studies have linked short-term sleep deprivations to changes in glucose metabolism, nervous system activity, or hormonal factors that may lead to diabetes.[34]
Dietary factors also influence the risk of developing type 2 diabetes. Consumption of sugar-sweetened drinks in excess is associated with an increased risk.[35][36] The type of fats in the diet are important, with saturated fats and trans fatty acids increasing the risk, and polyunsaturated and monounsaturated fat decreasing the risk.[28] Eating a lot of white rice appears to play a role in increasing risk.[37] A lack of exercise is believed to cause 7% of cases.[38] Persistent organic pollutants may also play a role.[39]
Genetics
Most cases of diabetes involve many genes, with each being a small contributor to an increased probability of becoming a type 2 diabetic.[10] The proportion of diabetes that is inherited is estimated at 72%.[40] More than 36 genes and 80 single nucleotide polymorphisms (SNPs) had been found that contribute to the risk of type 2 diabetes.[41][42] All of these genes together still only account for 10% of the total heritable component of the disease.[41] The TCF7L2 allele, for example, increases the risk of developing diabetes by 1.5 times and is the greatest risk of the common genetic variants.[13] Most of the genes linked to diabetes are involved in pancreatic beta cell functions.[13]
There are a number of rare cases of diabetes that arise due to an abnormality in a single gene (known as monogenic forms of diabetes or "other specific types of diabetes").[10][13] These include maturity onset diabetes of the young (MODY), Donohue syndrome, and Rabson–Mendenhall syndrome, among others.[10] Maturity onset diabetes of the young constitute 1–5% of all cases of diabetes in young people.[43]
Epigenetics
Epigenetic regulation occurs at multiple levels including (1) direct methylation of cytosine and adenine residues in DNA, (2) covalent modification of histone proteins in chromatin, and (3) action of non coding microRNAs (for other examples, see Wikipedia article “Epigenetics”). On November 17-19, 2017, the American Diabetes Association held a research symposium entitled “Epigenetics and Epigenomics: Implications for Diabetes and Obesity.” As a result of this symposium, an overview of the state of the field was presented in which it was noted that over 1,000 research articles have been published that address the intersection of diabetes and epigenetics or epigenomics.[44] The current state of knowledge in this field is addressed the Wikipedia article “Epigenetics of diabetes Type 2.”
Medical conditions
There are a number of medications and other health problems that can predispose to diabetes.[45] Some of the medications include: glucocorticoids, thiazides, beta blockers, atypical antipsychotics,[46] and statins.[47] Those who have previously had gestational diabetes are at a higher risk of developing type 2 diabetes.[23] Other health problems that are associated include: acromegaly, Cushing's syndrome, hyperthyroidism, pheochromocytoma, and certain cancers such as glucagonomas.[45] Individuals with cancer may be at a higher risk of mortality if they also have diabetes.[48] Testosterone deficiency is also associated with type 2 diabetes.[49][50] Eating disorders may also interact with type 2 diabetes, with bulimia nervosa increasing the risk and anorexia nervosa decreasing it.[51]
Pathophysiology
Type 2 diabetes is due to insufficient insulin production from beta cells in the setting of insulin resistance.[13] Insulin resistance, which is the inability of cells to respond adequately to normal levels of insulin, occurs primarily within the muscles, liver, and fat tissue.[52] In the liver, insulin normally suppresses glucose release. However, in the setting of insulin resistance, the liver inappropriately releases glucose into the blood.[10] The proportion of insulin resistance versus beta cell dysfunction differs among individuals, with some having primarily insulin resistance and only a minor defect in insulin secretion and others with slight insulin resistance and primarily a lack of insulin secretion.[13]
Other potentially important mechanisms associated with type 2 diabetes and insulin resistance include: increased breakdown of lipids within fat cells, resistance to and lack of incretin, high glucagon levels in the blood, increased retention of salt and water by the kidneys, and inappropriate regulation of metabolism by the central nervous system.[10] However, not all people with insulin resistance develop diabetes since an impairment of insulin secretion by pancreatic beta cells is also required.[13]
In the early stages of insulin resistance, the mass of beta cells expands, increasing the output of insulin to compensate for the insulin insensitivity.[53] But when type 2 diabetes has become manifest, a type 2 diabetic will have lost about half of their beta cells.[53] Fatty acids in the beta cells activate FOXO1, resulting in apoptosis of the beta cells.[53]
The causes of the aging-related insulin resistance seen in obesity and in type 2 diabetes are uncertain. Effects of intracellular lipid metabolism and ATP production in liver and muscle cells may contribute to insulin resistance.[54] New evidence also points to a role of a brain region called the hypothalamus in the development of insulin resistance. For one thing, a gene called Dusp8 is linked with an increased risk for diabetes.[55] This gene codes for a protein that regulates neuronal signaling in the hypothalamus. Also, infusions into the hypothalamus of a hormone called leptin normalize blood glucose and diminish insulin resistance in diabetic animals.[56] Activation of hypothalamic cells by leptin has an important role in maintaining normal levels of blood glucose. Thus, both the endocrine cells of the pancreas AND cells in the hypothalamus may have a role in the etiology of type 2 diabetes.
Hypothalamic cells regulate blood glucose via projections to the autonomic nervous system. Autonomic innervation of liver and muscle cells stimulates an increased uptake of glucose. In diabetic humans, the control of blood glucose by the autonomic nervous system is abnormal.[57] Leptin-sensitive, glucose regulating neurons become resistant to leptin during aging or during exposure to a high-fat diet. These leptin resistant neurons fail to restrain food intake, obesity, and blood glucose. The reasons for this lowered responsiveness to leptin are uncertain and are part of the puzzle of the causes of type 2 diabetes.[58]
Blood glucose levels can also be normalized in diabetic rodents by a single intrahypothalamic infusion of Fibroblast Growth Factor 1 (FGF1), an effect that persists for months even in severely diabetic animals. This remarkable cure of diabetes is accomplished by a stimulation of accessory brain cells called astrocytes.[59][60] Hypothalamic astrocytes that produce Fatty Acid Binding Protein 7 (FABP7) are targets of FGF1; these cells are also in close contact with leptin-sensitive neurons, influence their function, and regulate leptin sensitivity.[61][62] An abnormal function of FABP7+ astrocytes thus may contribute to the resistance to leptin and insulin that appear during aging and during exposure to high-fat diets.
During aging, FABP7+ astrocytes develop cytoplasmic granules derived from degenerating mitochondria. This mitochondrial degeneration is partly due to the oxidative stress of the heightened amounts of fatty acids that are taken up by these cells and oxidized within mitochondria.[63][64] A pathological degeneration of mitochondria in these cells may compromise their normal functions and contribute to abnormalities in the control of blood glucose by the hypothalamus.
Diagnosis
| Condition | 2-hour glucose | Fasting glucose | HbA1c | |||
|---|---|---|---|---|---|---|
| Unit | mmol/L | mg/dL | mmol/L | mg/dL | mmol/mol | DCCT % |
| Normal | < 7.8 | < 140 | < 6.1 | < 110 | < 42 | < 6.0 |
| Impaired fasting glycaemia | < 7.8 | < 140 | 6.1–7.0 | 110–125 | 42–46 | 6.0–6.4 |
| Impaired glucose tolerance | ≥ 7.8 | ≥ 140 | < 7.0 | < 126 | 42–46 | 6.0–6.4 |
| Diabetes mellitus | ≥ 11.1 | ≥ 200 | ≥ 7.0 | ≥ 126 | ≥ 48 | ≥ 6.5 |
The World Health Organization definition of diabetes (both type 1 and type 2) is for a single raised glucose reading with symptoms, otherwise raised values on two occasions, of either:[67]
- fasting plasma glucose ≥ 7.0 mmol/L (126 mg/dL)
- or
- with a glucose tolerance test, two hours after the oral dose a plasma glucose ≥ 11.1 mmol/L (200 mg/dL)
A random blood sugar of greater than 11.1 mmol/L (200 mg/dL) in association with typical symptoms[23] or a glycated hemoglobin (HbA1c) of ≥ 48 mmol/mol (≥ 6.5 DCCT %) is another method of diagnosing diabetes.[10] In 2009 an International Expert Committee that included representatives of the American Diabetes Association (ADA), the International Diabetes Federation (IDF), and the European Association for the Study of Diabetes (EASD) recommended that a threshold of ≥ 48 mmol/mol (≥ 6.5 DCCT %) should be used to diagnose diabetes.[68] This recommendation was adopted by the American Diabetes Association in 2010.[69] Positive tests should be repeated unless the person presents with typical symptoms and blood sugars >11.1 mmol/L (>200 mg/dL).[68]
| Diabetes mellitus | Prediabetes | |
|---|---|---|
| HbA1c | ≥6.5% | 5.7-6.4% |
| Fasting glucose | ≥126 mg/dL | 100-125 mg/dL |
| 2h glucose | ≥200 mg/dL | 140-199 mg/dL |
| Random glucose with classic symptoms | ≥200 mg/dL | Not available |
Threshold for diagnosis of diabetes is based on the relationship between results of glucose tolerance tests, fasting glucose or HbA1c and complications such as retinal problems.[10] A fasting or random blood sugar is preferred over the glucose tolerance test, as they are more convenient for people.[10] HbA1c has the advantages that fasting is not required and results are more stable but has the disadvantage that the test is more costly than measurement of blood glucose.[71] It is estimated that 20% of people with diabetes in the United States do not realize that they have the disease.[10]
Type 2 diabetes is characterized by high blood glucose in the context of insulin resistance and relative insulin deficiency.[72] This is in contrast to type 1 diabetes in which there is an absolute insulin deficiency due to destruction of islet cells in the pancreas and gestational diabetes that is a new onset of high blood sugars associated with pregnancy.[13] Type 1 and type 2 diabetes can typically be distinguished based on the presenting circumstances.[68] If the diagnosis is in doubt antibody testing may be useful to confirm type 1 diabetes and C-peptide levels may be useful to confirm type 2 diabetes,[73] with C-peptide levels normal or high in type 2 diabetes, but low in type 1 diabetes.[74]
Screening
No major organization recommends universal screening for diabetes as there is no evidence that such a program improve outcomes.[75][76] Screening is recommended by the United States Preventive Services Task Force (USPSTF) in adults without symptoms whose blood pressure is greater than 135/80 mmHg.[77] For those whose blood pressure is less, the evidence is insufficient to recommend for or against screening.[77] There is no evidence that it changes the risk of death in this group of people.[76] They also recommend screening among those who are overweight and between the ages of 40 and 70.[78]
The World Health Organization recommends testing those groups at high risk[75] and in 2014 the USPSTF is considering a similar recommendation.[79] High-risk groups in the United States include: those over 45 years old; those with a first degree relative with diabetes; some ethnic groups, including Hispanics, African-Americans, and Native-Americans; a history of gestational diabetes; polycystic ovary syndrome; excess weight; and conditions associated with metabolic syndrome.[23] The American Diabetes Association recommends screening those who have a BMI over 25 (in people of Asian descent screening is recommended for a BMI over 23).[80]
A Cochrane Systematic Review looking at the effects of screening on all-cause and diabetes-related mortality did not show any benefits in these outcomes for either screening or not-screening. This was based on only one included study, so no conclusions can be made on the benefits of screening, or lack thereof.[81] This same review did not assess the effects on other outcomes such as adverse effects, incidence of type 2 diabetes, HbA1c or socioeconomic effects.[81]
In the UK, NICE guidelines suggest taking action to prevent diabetes for people with a body mass index (BMI) of 30. For people of Black African, African-Caribbean, South Asian and Chinese descent the recommendation to start prevention starts at the BMI of 27,5.[82] A study based on a large sample of people in England suggest even lower BMIs for certain ethnic groups for the start of prevention, for example 24 in South Asian and 21 in Bangladeshi populations.[83][84]
Prevention
Onset of type 2 diabetes can be delayed or prevented through proper nutrition and regular exercise.[85][86] Intensive lifestyle measures may reduce the risk by over half.[25][87] The benefit of exercise occurs regardless of the person's initial weight or subsequent weight loss.[88] High levels of physical activity reduce the risk of diabetes by about 28%.[89] Evidence for the benefit of dietary changes alone, however, is limited,[90] with some evidence for a diet high in green leafy vegetables[91] and some for limiting the intake of sugary drinks.[92] There is an association between higher intake of sugar-sweetened fruit juice and diabetes, but no evidence of an association with 100% fruit juice.[93] A 2019 review found evidence of benefit from dietary fiber.[94]
In those with impaired glucose tolerance, a 2019 systematic review found moderate-quality evidence that Metformin, when compared to diet and exercise or a placebo intervention, appeared to delay or reduce the risk of developing type 2 diabetes.[95] This same review found moderate-quality evidence that when compared to intensive diet and exercise, Metformin did not reduce risk of developing type 2 diabetes, as well as very low-quality evidence that combining Metformin with intensive diet and exercise does not appear to have any effect on risk of developing type 2 diabetes when compared to intensive diet and exercise alone.[95] This systematic review only found one suitable trial comparing Metformin with Sulphonylurea in reducing risk of type 2 diabetes but it did not report any patient-relevant outcomes.[95]
A 2017 review found that, long term, lifestyle changes decreased the risk by 28%, while medication does not reduce risk after withdrawal.[96] While low vitamin D levels are associated with an increased risk of diabetes, correcting the levels by supplementing vitamin D3 does not improve that risk.[97]
Management
Management of type 2 diabetes focuses on lifestyle interventions, lowering other cardiovascular risk factors, and maintaining blood glucose levels in the normal range.[25] Self-monitoring of blood glucose for people with newly diagnosed type 2 diabetes may be used in combination with education,[98] although the benefit of self-monitoring in those not using multi-dose insulin is questionable.[25] In those who do not want to measure blood levels, measuring urine levels may be done.[99] Managing other cardiovascular risk factors, such as hypertension, high cholesterol, and microalbuminuria, improves a person's life expectancy.[25] Decreasing the systolic blood pressure to less than 140 mmHg is associated with a lower risk of death and better outcomes.[100] Intensive blood pressure management (less than 130/80 mmHg) as opposed to standard blood pressure management (less than 140-160 mmHg systolic to 85–100 mmHg diastolic) results in a slight decrease in stroke risk but no effect on overall risk of death.[101]
Intensive blood sugar lowering (HbA1c<6%) as opposed to standard blood sugar lowering (HbA1c of 7–7.9%) does not appear to change mortality.[102][103] The goal of treatment is typically an HbA1c of 7 to 8% or a fasting glucose of less than 7.2 mmol/L (130 mg/dL); however these goals may be changed after professional clinical consultation, taking into account particular risks of hypoglycemia and life expectancy.[80][104][105] Hypoglycemia is associated with adverse outcomes in older people with type 2 diabetes.[106] Despite guidelines recommending that intensive blood sugar control be based on balancing immediate harms with long-term benefits, many people – for example people with a life expectancy of less than nine years who will not benefit, are over-treated.[107]
It is recommended that all people with type 2 diabetes get regular eye examinations.[13] There is weak evidence suggesting that treating gum disease by scaling and root planing may result in a small short-term improvement in blood sugar levels for people with diabetes.[108][needs update] There is no evidence to suggest that this improvement in blood sugar levels is maintained longer than four months.[108] There is also not enough evidence to determine if medications to treat gum disease are effective at lowering blood sugar levels.[108]
Lifestyle
Exercise
A proper diet and regular exercise are foundations of diabetic care,[23] with one review indicating that a greater amount of exercise improved outcomes.[109] Regular exercise may improve blood sugar control, decrease body fat content, and decrease blood lipid levels.[110]
Diet
A diabetic diet which includes calorie restriction to promote weight loss is generally recommended.[111][70] Other recommendations include emphasizing intake of fruits, vegetables, reduced saturated fat and low-fat dairy products, and with a macronutrient intake tailored to the individual, to distribute calories and carbohydrates throughout the day.[70][112] Several diets may be effective such as the Dietary Approaches to Stop Hypertension (DASH), Mediterranean diet, low-fat diet, or monitored carbohydrate diets such as a low carbohydrate diet.[70][113][114] Viscous fiber supplements may be useful in those with diabetes.[115]
Vegetarian diets in general have been related to lower diabetes risk, but do not offer advantages compared with diets which allow moderate amounts of animal products.[116] There is not enough evidence to suggest that cinnamon improves blood sugar levels in people with type 2 diabetes.[117] A 2021 review showed that consumption of tree nuts (walnuts, almonds, and hazelnuts) reduced fasting blood glucose in diabetic people.[118]
Culturally appropriate education may help people with type 2 diabetes control their blood sugar levels for up to 24 months.[119] There is not enough evidence to determine if lifestyle interventions affect mortality in those who already have type 2 diabetes.[87]
As of 2015[update], there is insufficient data to recommend nonnutritive sweeteners, which may help reduce caloric intake.[120]
An elevated intake of microbiota-accessible carbohydrates can help reducing the effects of T2D.[121]
A 2022 umbrella review concluded that, red and white meat consumption is associated with an increased risk contrary to dairy products which have a diminished risk.[122]
Medications

Blood sugar control
There are several classes of anti-diabetic medications available. Metformin is generally recommended as a first line treatment as there is some evidence that it decreases mortality;[7][25][123] however, this conclusion is questioned.[124] Metformin should not be used in those with severe kidney or liver problems.[23]
A second oral agent of another class or insulin may be added if metformin is not sufficient after three months.[104] Other classes of medications include: sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, SGLT2 inhibitors, and glucagon-like peptide-1 analogs.[104] As of 2015 there was no significant difference between these agents.[104] A 2018 review found that SGLT2 inhibitors and GLP-1 agonists, but not DPP-4 inhibitors, were associated with lower mortality than placebo or no treatment.[125]
Rosiglitazone, a thiazolidinedione, has not been found to improve long-term outcomes even though it improves blood sugar levels.[126] Additionally it is associated with increased rates of heart disease and death.[127]
The effects of Plioglitazone have been compared in a Cochrane systematic review to that of other blood sugar lowering-medicine, including metformin, acarbose, and repaglinide, not showing any benefit in reducing the chance of developing type 2 diabetes in people at risk.[128] It did, however, show reduction of risk of developing type 2 diabetes when compared to a placebo or to no treatment.[128] These results should be interpreted considering that most of the data of the studies included in this review were of low or very-low certainty.
Injections of insulin may either be added to oral medication or used alone.[25] Most people do not initially need insulin.[13] When it is used, a long-acting formulation is typically added at night, with oral medications being continued.[23][25] Doses are then increased to effect (blood sugar levels being well controlled).[25] When nightly insulin is insufficient, twice daily insulin may achieve better control.[23] The long acting insulins glargine and detemir are equally safe and effective,[129] and do not appear much better than neutral protamine Hagedorn (NPH) insulin, but as they are significantly more expensive, they are not cost effective as of 2010.[130] In those who are pregnant, insulin is generally the treatment of choice.[23]
Blood pressure lowering
Many international guidelines recommend blood pressure treatment targets that are lower than 140/90 mmHg for people with diabetes.[131] However, there is only limited evidence regarding what the lower targets should be. A 2016 systematic review found potential harm to treating to targets lower than 140 mmHg,[132] and a subsequent review in 2019 found no evidence of additional benefit from blood pressure lowering to between 130–140mmHg, although there was an increased risk of adverse events.[133]
2015 American Diabetes Association recommendations are that people with diabetes and albuminuria should receive an inhibitor of the renin-angiotensin system to reduce the risks of progression to end-stage renal disease, cardiovascular events, and death.[70] There is some evidence that angiotensin converting enzyme inhibitors (ACEIs) are superior to other inhibitors of the renin-angiotensin system such as angiotensin receptor blockers (ARBs),[134] or aliskiren in preventing cardiovascular disease.[135] Although a more recent review found similar effects of ACEIs and ARBs on major cardiovascular and renal outcomes.[136] There is no evidence that combining ACEIs and ARBs provides additional benefits.[136]
Other
The use of aspirin to prevent cardiovascular disease in diabetes is controversial.[70] Aspirin is recommended in people at high risk of cardiovascular disease, however routine use of aspirin has not been found to improve outcomes in uncomplicated diabetes.[137] 2015 American Diabetes Association recommendations for aspirin use (based on expert consensus or clinical experience) are that low-dose aspirin use is reasonable in adults with diabetes who are at intermediate risk of cardiovascular disease (10-year cardiovascular disease risk, 5–10%).[70]
Vitamin D supplementation to people with type 2 diabetes may improve markers of insulin resistance and HbA1c.[138]
Sharing their electronic health records with people who have type 2 diabetes helps them to reduce their blood sugar levels. It is a way of helping people understand their own health condition and involving them actively in its management.[139][140]
Surgery
Weight loss surgery in those who are obese is an effective measure to treat diabetes.[141] Many are able to maintain normal blood sugar levels with little or no medication following surgery[142] and long-term mortality is decreased.[143] There however is some short-term mortality risk of less than 1% from the surgery.[144] The body mass index cutoffs for when surgery is appropriate are not yet clear.[143] It is recommended that this option be considered in those who are unable to get both their weight and blood sugar under control.[145][146]
Epidemiology
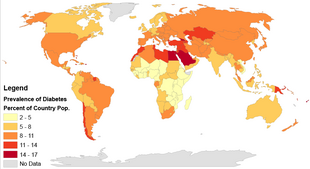
The International Diabetes Federation estimates nearly 537 million people lived with diabetes worldwide in 2021,[147] 90–95% of whom have type 2 diabetes.[148] Diabetes is common both in the developed and the developing world.[10] It remains uncommon, however, in the least developed countries.[13]
Women seem to be at a greater risk as do certain ethnic groups,[10][149] such as South Asians, Pacific Islanders, Latinos, and Native Americans.[23] This may be due to enhanced sensitivity to a Western lifestyle in certain ethnic groups.[150] Traditionally considered a disease of adults, type 2 diabetes is increasingly diagnosed in children in parallel with rising obesity rates.[10] Type 2 diabetes is now diagnosed as frequently as type 1 diabetes in teenagers in the United States.[13]
Rates of diabetes in 1985 were estimated at 30 million, increasing to 135 million in 1995 and 217 million in 2005.[18] This increase is believed to be primarily due to the global population aging, a decrease in exercise, and increasing rates of obesity.[18] The five countries with the greatest number of people with diabetes as of 2000 are India having 31.7 million, China 20.8 million, the United States 17.7 million, Indonesia 8.4 million, and Japan 6.8 million.[151] It is recognized as a global epidemic by the World Health Organization.[1]
History
Diabetes is one of the first diseases described[21] with an Egyptian manuscript from c. 1500 BCE mentioning "too great emptying of the urine."[152] The first described cases are believed to be of type 1 diabetes.[152] Indian physicians around the same time identified the disease and classified it as madhumeha or honey urine noting that the urine would attract ants.[152] The term "diabetes" or "to pass through" was first used in 230 BCE by the Greek Apollonius Memphites.[152] The disease was rare during the time of the Roman empire with Galen commenting that he had only seen two cases during his career.[152]
Type 1 and type 2 diabetes were identified as separate conditions for the first time by the Indian physicians Sushruta and Charaka in 400–500 AD with type 1 associated with youth and type 2 with being overweight.[152] Effective treatment was not developed until the early part of the 20th century when the Canadians Frederick Banting and Charles Best discovered insulin in 1921 and 1922.[152] This was followed by the development of the long acting NPH insulin in the 1940s.[152]
In 1916, Elliot Joslin proposed that in people with diabetes, periods of fasting are helpful.[153] Subsequent research has supported this, and weight loss is a first line treatment in type 2 diabetes.[153]
Research
Researchers developed the Diabetes Severity Score (DISSCO), a tool that might better than the standard blood test at identify if a person's condition is declining. It uses a computer algorithm to analyse data from anonymised electronic patient records and produces a score based on 34 indicators.[154][155]
References
- ^ a b c d e f g h i j k "Diabetes Fact sheet N°312". World Health Organization. August 2011. Archived from the original on 26 August 2013. Retrieved 2012-01-09.
- ^ "Diabetes Blue Circle Symbol". International Diabetes Federation. 17 March 2006. Archived from the original on 5 August 2007.
- ^ a b c d e "Diagnosis of Diabetes and Prediabetes". National Institute of Diabetes and Digestive and Kidney Diseases. June 2014. Archived from the original on 6 March 2016. Retrieved 10 February 2016.
- ^ a b Pasquel FJ, Umpierrez GE (November 2014). "Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment". Diabetes Care. 37 (11): 3124–31. doi:10.2337/dc14-0984. PMC 4207202. PMID 25342831.
- ^ a b Fasanmade OA, Odeniyi IA, Ogbera AO (June 2008). "Diabetic ketoacidosis: diagnosis and management". African Journal of Medicine and Medical Sciences. 37 (2): 99–105. PMID 18939392.
- ^ a b c d e f g "Causes of Diabetes". National Institute of Diabetes and Digestive and Kidney Diseases. June 2014. Archived from the original on 2 February 2016. Retrieved 10 February 2016.
- ^ a b c Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S (June 2016). "Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis". Annals of Internal Medicine. 164 (11): 740–51. doi:10.7326/M15-2650. PMID 27088241. S2CID 32016657.
- ^ a b Cetinkunar S, Erdem H, Aktimur R, Sozen S (June 2015). "Effect of bariatric surgery on humoral control of metabolic derangements in obese patients with type 2 diabetes mellitus: How it works". World Journal of Clinical Cases. 3 (6): 504–9. doi:10.12998/wjcc.v3.i6.504. PMC 4468896. PMID 26090370.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Krentz AJ, Bailey CJ (February 2005). "Oral antidiabetic agents: current role in type 2 diabetes mellitus". Drugs. 65 (3): 385–411. doi:10.2165/00003495-200565030-00005. PMID 15669880. S2CID 29670619.
- ^ a b c d e f g h i j k l m n o p q r Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. (2011). Williams textbook of endocrinology (12th ed.). Philadelphia: Elsevier/Saunders. pp. 1371–1435. ISBN 978-1-4377-0324-5.
- ^ a b Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators) (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ MacKay I, Rose N, eds. (2014). The Autoimmune Diseases. Academic Press. p. 575. ISBN 978-0-123-84929-8. OCLC 965646175.
- ^ a b c d e f g h i j k l m n o p q Gardner DG, Shoback D, eds. (2011). "Chapter 17: Pancreatic hormones & diabetes mellitus". Greenspan's basic & clinical endocrinology (9th ed.). New York: McGraw-Hill Medical. ISBN 978-0-07-162243-1. OCLC 613429053.
- ^ Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D (July 2005). Saenz A (ed.). "Metformin monotherapy for type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews (3): CD002966. doi:10.1002/14651858.CD002966.pub3. PMID 16034881. (Retracted)
- ^ Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD (January 2012). "Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin". The Cochrane Database of Systematic Reviews. 1: CD005060. doi:10.1002/14651858.CD005060.pub3. hdl:1871/48558. PMID 22258959. S2CID 205176936.
- ^ Ganguly S, Tan HC, Lee PC, Tham KW (April 2015). "Metabolic bariatric surgery and type 2 diabetes mellitus: an endocrinologist's perspective". Journal of Biomedical Research. 29 (2): 105–11. doi:10.7555/JBR.29.20140127. PMC 4389109. PMID 25859264.
- ^ Moscou S (2013). "Getting the word out: advocacy, social marketing, and policy development and enforcement". In Truglio-Londrigan M, Lewenson SB (eds.). Public health nursing: practicing population-based care (2nd ed.). Burlington, MA: Jones & Bartlett Learning. p. 317. ISBN 978-1-4496-4660-8. OCLC 758391750.
- ^ a b c Smyth S, Heron A (January 2006). "Diabetes and obesity: the twin epidemics". Nature Medicine. 12 (1): 75–80. doi:10.1038/nm0106-75. PMID 16397575. S2CID 1042625.
- ^ Tfayli H, Arslanian S (March 2009). "Pathophysiology of type 2 diabetes mellitus in youth: the evolving chameleon". Arquivos Brasileiros de Endocrinologia e Metabologia. 53 (2): 165–74. doi:10.1590/s0004-27302009000200008. PMC 2846552. PMID 19466209.
- ^ Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, Lawrence JM, Liese AD, Liu LL, Mayer-Davis EJ, Rodriguez BL, Standiford D (December 2012). "Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth". Diabetes Care. 35 (12): 2515–20. doi:10.2337/dc12-0669. PMC 3507562. PMID 23173134.
- ^ a b Leutholtz BC, Ripoll I (2011). "Diabetes". Exercise and disease management (2nd ed.). Boca Raton: CRC Press. p. 25. ISBN 978-1-4398-2759-8. OCLC 725919496.
- ^ Zaccardi F, Webb DR, Yates T, Davies MJ (February 2016). "Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective". Postgraduate Medical Journal. 92 (1084): 63–9. doi:10.1136/postgradmedj-2015-133281. PMID 26621825. S2CID 28169759.
- ^ a b c d e f g h i j k Vijan S (March 2010). "In the clinic. Type 2 diabetes". Annals of Internal Medicine. 152 (5): ITC31–15, quiz ITC316. doi:10.7326/0003-4819-152-5-201003020-01003. PMID 20194231. S2CID 207535925.
- ^ Rathee M, Prachi J (2019). "Ageusia". StatPearls. StatPearls Publishing.
- ^ a b c d e f g h i j Ripsin CM, Kang H, Urban RJ (January 2009). "Management of blood glucose in type 2 diabetes mellitus". American Family Physician. 79 (1): 29–36. PMID 19145963.
- ^ Pasquier F (October 2010). "Diabetes and cognitive impairment: how to evaluate the cognitive status?". Diabetes & Metabolism. 36 (Suppl 3): S100-5. doi:10.1016/S1262-3636(10)70475-4. PMID 21211730.
- ^ Akinpelu OV, Mujica-Mota M, Daniel SJ (March 2014). "Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis". The Laryngoscope. 124 (3): 767–776. doi:10.1002/lary.24354. PMID 23945844. S2CID 25569962.
- ^ a b Risérus U, Willett WC, Hu FB (January 2009). "Dietary fats and prevention of type 2 diabetes". Progress in Lipid Research. 48 (1): 44–51. doi:10.1016/j.plipres.2008.10.002. PMC 2654180. PMID 19032965.
- ^ Hilawe EH, Yatsuya H, Kawaguchi L, Aoyama A (September 2013). "Differences by sex in the prevalence of diabetes mellitus, impaired fasting glycaemia and impaired glucose tolerance in sub-Saharan Africa: a systematic review and meta-analysis". Bulletin of the World Health Organization. 91 (9): 671–682D. doi:10.2471/BLT.12.113415. PMC 3790213. PMID 24101783.
- ^ Christian P, Stewart CP (March 2010). "Maternal micronutrient deficiency, fetal development, and the risk of chronic disease". The Journal of Nutrition. 140 (3): 437–45. doi:10.3945/jn.109.116327. PMID 20071652.
- ^ Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al. (July 2016). "Human gut microbes impact host serum metabolome and insulin sensitivity". Nature. 535 (7612): 376–81. Bibcode:2016Natur.535..376P. doi:10.1038/nature18646. PMID 27409811. S2CID 4459808.[permanent dead link]
- ^ Abdullah A, Peeters A, de Courten M, Stoelwinder J (September 2010). "The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies". Diabetes Research and Clinical Practice. 89 (3): 309–19. doi:10.1016/j.diabres.2010.04.012. PMID 20493574.
- ^ Pan A, Wang Y, Talaei M, Hu FB, Wu T (December 2015). "Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis". The Lancet. Diabetes & Endocrinology. 3 (12): 958–67. doi:10.1016/S2213-8587(15)00316-2. PMC 4656094. PMID 26388413.
- ^ a b Touma C, Pannain S (August 2011). "Does lack of sleep cause diabetes?". Cleveland Clinic Journal of Medicine. 78 (8): 549–58. doi:10.3949/ccjm.78a.10165. PMID 21807927. S2CID 45708828.
- ^ Malik VS, Popkin BM, Bray GA, Després JP, Hu FB (March 2010). "Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk". Circulation. 121 (11): 1356–64. doi:10.1161/CIRCULATIONAHA.109.876185. PMC 2862465. PMID 20308626.
- ^ Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB (November 2010). "Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis". Diabetes Care. 33 (11): 2477–83. doi:10.2337/dc10-1079. PMC 2963518. PMID 20693348.
- ^ Hu EA, Pan A, Malik V, Sun Q (March 2012). "White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review". BMJ. 344: e1454. doi:10.1136/bmj.e1454. PMC 3307808. PMID 22422870.
- ^ Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT (July 2012). "Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy". Lancet. 380 (9838): 219–29. doi:10.1016/S0140-6736(12)61031-9. PMC 3645500. PMID 22818936.
- ^ Lind L, Lind PM (June 2012). "Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease?". Journal of Internal Medicine. 271 (6): 537–53. doi:10.1111/j.1365-2796.2012.02536.x. PMID 22372998. S2CID 41018361.
- ^ Willemsen G, Ward KJ, Bell CG, Christensen K, Bowden J, Dalgård C, et al. (December 2015). "The Concordance and Heritability of Type 2 Diabetes in 34,166 Twin Pairs From International Twin Registers: The Discordant Twin (DISCOTWIN) Consortium". Twin Research and Human Genetics. 18 (6): 762–771. doi:10.1017/thg.2015.83. PMID 26678054.
- ^ a b Herder C, Roden M (June 2011). "Genetics of type 2 diabetes: pathophysiologic and clinical relevance". European Journal of Clinical Investigation. 41 (6): 679–692. doi:10.1111/j.1365-2362.2010.02454.x. PMID 21198561. S2CID 43548816.
- ^ Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. (August 2016). "The genetic architecture of type 2 diabetes". Nature. 536 (7614): 41–47. Bibcode:2016Natur.536...41F. doi:10.1038/nature18642. PMC 5034897. PMID 27398621.
- ^ "Monogenic Forms of Diabetes: Neonatal Diabetes Mellitus and Maturity-onset Diabetes of the Young". National Diabetes Information Clearinghouse (NDIC). National Institute of Diabetes and Digestive and Kidney Diseases, NIH. March 2007. Archived from the original on 2008-07-04. Retrieved 2008-08-04.
- ^ Rosen ED, Kaestner KH, Natarajan R, Patti ME, Sallari R, Sander M, Susztak K. Epigenetics and Epigenomics: Implications for Diabetes and Obesity. Diabetes. 2018 Oct;67(10):1923-1931. doi: 10.2337/db18-0537. PMID: 30237160; PMCID: PMC6463748
- ^ a b Funnell MM, Anderson RM (2008). "Influencing self-management: from compliance to collaboration". In Bethel MN, Feinglos MA (eds.). Type 2 diabetes mellitus: an evidence-based approach to practical management. Contemporary endocrinology. Totowa, NJ: Humana Press. p. 462. ISBN 978-1-58829-794-5. OCLC 261324723.
- ^ Izzedine H, Launay-Vacher V, Deybach C, Bourry E, Barrou B, Deray G (November 2005). "Drug-induced diabetes mellitus". Expert Opinion on Drug Safety. 4 (6): 1097–1109. doi:10.1517/14740338.4.6.1097. PMID 16255667. S2CID 21532595.
- ^ Sampson UK, Linton MF, Fazio S (July 2011). "Are statins diabetogenic?". Current Opinion in Cardiology. 26 (4): 342–347. doi:10.1097/HCO.0b013e3283470359. PMC 3341610. PMID 21499090.
- ^ Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. (July 2010). "Diabetes and cancer: a consensus report". Diabetes Care (Professional society guidelines). 33 (7): 1674–1685. doi:10.2337/dc10-0666. PMC 2890380. PMID 20587728.
- ^ Saad F, Gooren L (March 2009). "The role of testosterone in the metabolic syndrome: a review". The Journal of Steroid Biochemistry and Molecular Biology. 114 (1–2): 40–43. doi:10.1016/j.jsbmb.2008.12.022. PMID 19444934. S2CID 22222112.
- ^ Farrell JB, Deshmukh A, Baghaie AA (2008). "Low testosterone and the association with type 2 diabetes". The Diabetes Educator. 34 (5): 799–806. doi:10.1177/0145721708323100. PMID 18832284.
- ^ Nieto-Martínez R, González-Rivas JP, Medina-Inojosa JR, Florez H (November 2017). "Are Eating Disorders Risk Factors for Type 2 Diabetes? A Systematic Review and Meta-analysis". Current Diabetes Reports (Systematic review and meta-analysis). 17 (12): 138. doi:10.1007/s11892-017-0949-1. PMID 29168047. S2CID 3688434.
- ^ Diabetes mellitus a guide to patient care. Philadelphia: Lippincott Williams & Wilkins. 2007. p. 15. ISBN 978-1-58255-732-8.
- ^ a b c Sun T, Han X (2019). "Death versus dedifferentiation: The molecular bases of beta cell mass reduction in type 2 diabetes". Seminars in Cell and Developmental Biology. 103: 76–82. doi:10.1016/j.semcdb.2019.12.002. PMID 31831356. S2CID 209341381.
- ^ Reed J, Bain S, Kanamarlapudi V (August 2021). "A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives". Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 14: 3567–3602. doi:10.2147/DMSO.S319895. PMC 8369920. PMID 34413662.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Schriever SC, Kabra DG, Pfuhlmann K, Baumann P, Baumgart EV, Nagler J, et al. (November 2020). "Type 2 diabetes risk gene Dusp8 regulates hypothalamic Jnk signaling and insulin sensitivity". The Journal of Clinical Investigation. 130 (11): 6093–6108. doi:10.1172/JCI136363. PMC 7598066. PMID 32780722.
- ^ German JP, Wisse BE, Thaler JP, Oh-I S, Sarruf DA, Ogimoto K, et al. (July 2010). "Leptin deficiency causes insulin resistance induced by uncontrolled diabetes". Diabetes. 59 (7): 1626–1634. doi:10.2337/db09-1918. PMC 2889761. PMID 20424233.
- ^ Lundqvist MH, Almby K, Wiklund U, Abrahamsson N, Kamble PG, Pereira MJ, Eriksson JW (March 2021). "Altered hormonal and autonomic nerve responses to hypo- and hyperglycaemia are found in overweight and insulin-resistant individuals and may contribute to the development of type 2 diabetes". Diabetologia. 64 (3): 641–655. doi:10.1007/s00125-020-05332-z. PMC 7864814. PMID 33241460.
- ^ Salazar J, Chávez-Castillo M, Rojas J, Ortega A, Nava M, Pérez J, et al. (2020-07-23). "Is "Leptin Resistance" Another Key Resistance to Manage Type 2 Diabetes?". Current Diabetes Reviews. 16 (7): 733–749. doi:10.2174/1573399816666191230111838. PMID 31886750. S2CID 209510992.
- ^ Alonge KM, D'Alessio DA, Schwartz MW (January 2021). "Brain control of blood glucose levels: implications for the pathogenesis of type 2 diabetes". Diabetologia. 64 (1): 5–14. doi:10.1007/s00125-020-05293-3. PMC 7718404. PMID 33043401.
- ^ Bentsen MA, Rausch DM, Mirzadeh Z, Muta K, Scarlett JM, Brown JM, et al. (September 2020). "Transcriptomic analysis links diverse hypothalamic cell types to fibroblast growth factor 1-induced sustained diabetes remission". Nature Communications. 11 (1): 4458. Bibcode:2020NatCo..11.4458B. doi:10.1038/s41467-020-17720-5. PMC 7477234. PMID 32895383.
- ^ Adlanmerini M, Nguyen HC, Krusen BM, Teng CW, Geisler CE, Peed LC, et al. (January 2021). "Hypothalamic REV-ERB nuclear receptors control diurnal food intake and leptin sensitivity in diet-induced obese mice". The Journal of Clinical Investigation. 131 (1): e140424. doi:10.1172/JCI140424. PMC 7773391. PMID 33021965.
- ^ Yasumoto Y, Miyazaki H, Ogata M, Kagawa Y, Yamamoto Y, Islam A, et al. (December 2018). "Glial Fatty Acid-Binding Protein 7 (FABP7) Regulates Neuronal Leptin Sensitivity in the Hypothalamic Arcuate Nucleus". Molecular Neurobiology. 55 (12): 9016–9028. doi:10.1007/s12035-018-1033-9. PMID 29623545. S2CID 4632807.
- ^ Young JK, Baker JH, Muller T (March 1996). "Immunoreactivity for brain-fatty acid binding protein in gomori-positive astrocytes". Glia. 16 (3): 218–226. doi:10.1002/(SICI)1098-1136(199603)16:3<218::AID-GLIA4>3.0.CO;2-Y. PMID 8833192. S2CID 9757285.
- ^ Schmidt SP, Corydon TJ, Pedersen CB, Vang S, Palmfeldt J, Stenbroen V, et al. (April 2011). "Toxic response caused by a misfolding variant of the mitochondrial protein short-chain acyl-CoA dehydrogenase". Journal of Inherited Metabolic Disease. 34 (2): 465–475. doi:10.1007/s10545-010-9255-7. PMC 3063561. PMID 21170680.
- ^ Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF consultation (PDF). Geneva: World Health Organization. 2006. p. 21. ISBN 978-92-4-159493-6.
- ^ Vijan S (March 2010). "In the clinic. Type 2 diabetes". Annals of Internal Medicine. 152 (5): ITC31-15, quiz ITC316. doi:10.7326/0003-4819-152-5-201003020-01003. PMID 20194231.
- ^ World Health Organization. "Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. Diagnosis and classification of diabetes mellitus". Archived from the original on 2007-05-29. Retrieved 2007-05-29.
- ^ a b c International Expert Committee (July 2009). "International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes". Diabetes Care. 32 (7): 1327–34. doi:10.2337/dc09-9033. PMC 2699715. PMID 19502545.
- ^ American Diabetes Association (January 2010). "Diagnosis and classification of diabetes mellitus". Diabetes Care. 33 (Supplement_1): S62-9. doi:10.2337/dc10-S062. PMC 2797383. PMID 20042775.
- ^ a b c d e f g Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, et al. (September 2015). "Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association". Diabetes Care (Professional society guidelines). 38 (9): 1777–1803. doi:10.2337/dci15-0012. PMC 4876675. PMID 26246459.
- ^ American Diabetes Association (January 2012). "Diagnosis and classification of diabetes mellitus". Diabetes Care. 35 (Suppl 1): S64-71. doi:10.2337/dc12-s064. PMC 3632174. PMID 22187472.
- ^ Kumar V, Fausto N, Abbas AK, Cotran RS, Robbins SL (2005). Robbins and Cotran Pathologic Basis of Disease (7th ed.). Philadelphia, Pa.: Saunders. pp. 1194–95. ISBN 978-0-7216-0187-8.
- ^ Diabetes mellitus a guide to patient care. Philadelphia: Lippincott Williams & Wilkins. 2007. p. 201. ISBN 978-1-58255-732-8.
- ^ Vivian EM, Blackorbay B (2013). "Chapter 13: Endocrine Disorders". In Lee M (ed.). Basic Skills in Interpreting Laboratory Data (5th ed.). Bethesda, MD: American Society of Health-System Pharmacists. ISBN 978-1-58528-345-3. OCLC 859778842.
- ^ a b Valdez R (July 2009). "Detecting undiagnosed type 2 diabetes: family history as a risk factor and screening tool". Journal of Diabetes Science and Technology. 3 (4): 722–6. doi:10.1177/193229680900300417. PMC 2769984. PMID 20144319.
- ^ a b Selph S, Dana T, Blazina I, Bougatsos C, Patel H, Chou R (June 2015). "Screening for type 2 diabetes mellitus: a systematic review for the U.S. Preventive Services Task Force". Annals of Internal Medicine. 162 (11): 765–76. doi:10.7326/M14-2221. PMID 25867111.
- ^ a b "Archived: Diabetes Mellitus (Type 2) in Adults: Screening". U.S. Preventive Services Task Force. June 2008. Archived from the original on 2014-02-07. Retrieved 2014-03-16.
- ^ Siu AL (December 2015). "Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: U.S. Preventive Services Task Force Recommendation Statement". Annals of Internal Medicine. 163 (11): 861–8. doi:10.7326/M15-2345. PMID 26501513.
- ^ "Draft Recommendation Statement Screening for Abnormal Glucose and Type 2 Diabetes Mellitus". U.S. Preventive Services Task Force. Archived from the original on 9 October 2014. Retrieved 7 October 2014.
- ^ a b "Standards of medical care in diabetes--2015: summary of revisions". Diabetes Care. 38 (38): S4. January 2015. doi:10.2337/dc15-S003. PMID 25537706.
- ^ a b Peer N, Balakrishna Y, Durao S (May 2020). "Screening for type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews. 5 (6): CD005266. doi:10.1002/14651858.cd005266.pub2. PMC 7259754. PMID 32470201.
- ^ "Diabetes: putting people at the heart of services". NIHR Evidence. National Institute for Health and Care Research. 2022-07-26. doi:10.3310/nihrevidence_52026.
- ^ "Are you at risk of diabetes? Research finds prevention should start at a different BMI for each ethnic group". NIHR Evidence (Plain English summary). National Institute for Health and Care Research. 2022-03-10. doi:10.3310/alert_48878.
- ^ Caleyachetty R, Barber TM, Mohammed NI, Cappuccio FP, Hardy R, Mathur R, et al. (July 2021). "Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study". The Lancet. Diabetes & Endocrinology. 9 (7): 419–426. doi:10.1016/S2213-8587(21)00088-7. PMC 8208895. PMID 33989535.
- ^ Raina Elley C, Kenealy T (December 2008). "Lifestyle interventions reduced the long-term risk of diabetes in adults with impaired glucose tolerance". Evidence-Based Medicine. 13 (6): 173. doi:10.1136/ebm.13.6.173. PMID 19043031. S2CID 26714233.
- ^ Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I, Figuls M, Metzendorf MI, Richter B (December 2017). "Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews. 2017 (12): CD003054. doi:10.1002/14651858.CD003054.pub4. PMC 6486271. PMID 29205264.
- ^ a b Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C (October 2013). "Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis". Annals of Internal Medicine. 159 (8): 543–551. doi:10.7326/0003-4819-159-8-201310150-00007. PMID 24126648.
- ^ O'Gorman DJ, Krook A (September 2011). "Exercise and the treatment of diabetes and obesity". The Medical Clinics of North America. 95 (5): 953–969. doi:10.1016/j.mcna.2011.06.007. PMID 21855702.
- ^ Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. (August 2016). "Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013". BMJ. 354: i3857. doi:10.1136/bmj.i3857. PMC 4979358. PMID 27510511.
- ^ Nield L, Summerbell CD, Hooper L, Whittaker V, Moore H (July 2008). Nield L (ed.). "Dietary advice for the prevention of type 2 diabetes mellitus in adults". The Cochrane Database of Systematic Reviews (3): CD005102. doi:10.1002/14651858.CD005102.pub2. hdl:10149/92337. PMID 18646120. S2CID 23039006. (Retracted)
- ^ Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ (August 2010). "Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis". BMJ. 341: c4229. doi:10.1136/bmj.c4229. PMC 2924474. PMID 20724400.
- ^ Schwingshackl L, Hoffmann G, Lampousi AM, Knüppel S, Iqbal K, Schwedhelm C, et al. (May 2017). "Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies". European Journal of Epidemiology. 32 (5): 363–375. doi:10.1007/s10654-017-0246-y. PMC 5506108. PMID 28397016.
- ^ Xi B, Li S, Liu Z, Tian H, Yin X, Huai P, et al. (2014). "Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis". PLOS ONE. 9 (3): e93471. Bibcode:2014PLoSO...993471X. doi:10.1371/journal.pone.0093471. PMC 3969361. PMID 24682091.
- ^ Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L (February 2019). "Carbohydrate quality and human health: a series of systematic reviews and meta-analyses". Lancet. 393 (10170): 434–445. doi:10.1016/S0140-6736(18)31809-9. PMID 30638909. S2CID 58632705.
- ^ a b c Madsen, Kasper S; Chi, Yuan; Metzendorf, Maria-Inti; Richter, Bernd; Hemmingsen, Bianca (2019-12-03). Cochrane Metabolic and Endocrine Disorders Group (ed.). "Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus". Cochrane Database of Systematic Reviews. 2019 (12). doi:10.1002/14651858.CD008558.pub2. PMC 6889926. PMID 31794067.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Haw JS, Galaviz KI, Straus AN, Kowalski AJ, Magee MJ, Weber MB, Wei J, Narayan KM, Ali MK (December 2017). "Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials". JAMA Internal Medicine. 177 (12): 1808–1817. doi:10.1001/jamainternmed.2017.6040. PMC 5820728. PMID 29114778.
- ^ Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA (October 2014). "Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis". The Journal of Clinical Endocrinology and Metabolism. 99 (10): 3551–60. doi:10.1210/jc.2014-2136. PMC 4483466. PMID 25062463.
- ^ Mannucci E, Giaccari A, Gallo M, Bonifazi A, Belén ÁD, Masini ML, et al. (February 2022). "Self-management in patients with type 2 diabetes: Group-based versus individual education. A systematic review with meta-analysis of randomized trails". Nutrition, Metabolism, and Cardiovascular Diseases. 32 (2): 330–336. doi:10.1016/j.numecd.2021.10.005. PMID 34893413. S2CID 244580173.
- ^ "Type 2 diabetes: The management of type 2 diabetes". May 2009. Archived from the original on 2015-05-22.
- ^ Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A (February 2015). "Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis". JAMA. 313 (6): 603–615. doi:10.1001/jama.2014.18574. PMID 25668264.
- ^ McBrien K, Rabi DM, Campbell N, Barnieh L, Clement F, Hemmelgarn BR, et al. (September 2012). "Intensive and Standard Blood Pressure Targets in Patients With Type 2 Diabetes Mellitus: Systematic Review and Meta-analysis". Archives of Internal Medicine. 172 (17): 1296–1303. doi:10.1001/archinternmed.2012.3147. PMID 22868819.
- ^ Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassaï B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C (July 2011). "Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials". BMJ. 343: d4169. doi:10.1136/bmj.d4169. PMC 3144314. PMID 21791495.
- ^ Webster MW (July 2011). "Clinical practice and implications of recent diabetes trials". Current Opinion in Cardiology. 26 (4): 288–93. doi:10.1097/HCO.0b013e328347b139. PMID 21577100. S2CID 20819316.
- ^ a b c d Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR (March 2015). "Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes". Diabetologia. 58 (3): 429–42. doi:10.1007/s00125-014-3460-0. PMID 25583541.
- ^ Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA (April 2018). "Hemoglobin A1c Targets for Glycemic Control With Pharmacologic Therapy for Nonpregnant Adults With Type 2 Diabetes Mellitus: A Guidance Statement Update From the American College of Physicians". Annals of Internal Medicine. 168 (8): 569–576. doi:10.7326/M17-0939. PMID 29507945.
- ^ Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. (May 2013). "Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society". Diabetes Care (Professional society guidelines). 36 (5): 1384–1395. doi:10.2337/dc12-2480. PMC 3631867. PMID 23589542.
- ^ Makam AN, Nguyen OK (January 2017). "An Evidence-Based Medicine Approach to Antihyperglycemic Therapy in Diabetes Mellitus to Overcome Overtreatment". Circulation. 135 (2): 180–195. doi:10.1161/CIRCULATIONAHA.116.022622. PMC 5502688. PMID 28069712.
- ^ a b c Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, Stevenson B, Furness S, Iheozor-Ejiofor Z (November 2015). "Treatment of periodontal disease for glycaemic control in people with diabetes mellitus". The Cochrane Database of Systematic Reviews. 2018 (11): CD004714. doi:10.1002/14651858.CD004714.pub3. PMC 6486035. PMID 26545069.
- ^ Smith AD, Crippa A, Woodcock J, Brage S (December 2016). "Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies". Diabetologia. 59 (12): 2527–2545. doi:10.1007/s00125-016-4079-0. PMC 6207340. PMID 27747395.
- ^ Thomas DE, Elliott EJ, Naughton GA (July 2006). "Exercise for type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews. 2009 (3): CD002968. doi:10.1002/14651858.CD002968.pub2. PMC 8989410. PMID 16855995. S2CID 25505640.
- ^ Davis N, Forbes B, Wylie-Rosett J (June 2009). "Nutritional strategies in type 2 diabetes mellitus". The Mount Sinai Journal of Medicine, New York. 76 (3): 257–268. doi:10.1002/msj.20118. PMID 19421969.
- ^ Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. (January 2014). "Nutrition therapy recommendations for the management of adults with diabetes". Diabetes Care (Professional society guidelines). 37 (Supplement_1): S120–S143. doi:10.2337/dc14-S120. PMID 24357208.
- ^ Thomas D, Elliott EJ (January 2009). Thomas D (ed.). "Low glycaemic index, or low glycaemic load, diets for diabetes mellitus". The Cochrane Database of Systematic Reviews (1): CD006296. doi:10.1002/14651858.CD006296.pub2. PMC 6486008. PMID 19160276.
- ^ Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, et al. (January 2015). "Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base". Nutrition. 31 (1): 1–13. doi:10.1016/j.nut.2014.06.011. PMID 25287761.
- ^ Jovanovski E, Khayyat R, Zurbau A, Komishon A, Mazhar N, Sievenpiper JL, et al. (May 2019). "Should Viscous Fiber Supplements Be Considered in Diabetes Control? Results From a Systematic Review and Meta-analysis of Randomized Controlled Trials". Diabetes Care. 42 (5): 755–766. doi:10.2337/dc18-1126. PMID 30617143. S2CID 58665219.
- ^ Glick-Bauer M, Yeh MC (October 2014). "The health advantage of a vegan diet: exploring the gut microbiota connection". Nutrients (Review). 6 (11): 4822–4838. doi:10.3390/nu6114822. PMC 4245565. PMID 25365383.
- ^ Leach MJ, Kumar S (September 2012). "Cinnamon for diabetes mellitus". The Cochrane Database of Systematic Reviews (9): CD007170. doi:10.1002/14651858.CD007170.pub2. PMC 6486047. PMID 22972104.
- ^ Muley A, Fernandez R, Ellwood L, Muley P, Shah M (May 2021). "Effect of tree nuts on glycemic outcomes in adults with type 2 diabetes mellitus: a systematic review". JBI Evidence Synthesis. 19 (5): 966–1002. doi:10.11124/JBISRIR-D-19-00397. PMID 33141798. S2CID 226250006.
- ^ Attridge M, Creamer J, Ramsden M, Cannings-John R, Hawthorne K (September 2014). "Culturally appropriate health education for people in ethnic minority groups with type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews (9): CD006424. doi:10.1002/14651858.CD006424.pub3. PMID 25188210.
- ^ Gardner, Christopher; Wylie-Rosett, Judith; Gidding, Samuel S.; Steffen, Lyn M.; Johnson, Rachel K.; Reader, Diane; Lichtenstein, Alice H. (1 August 2012). "Nonnutritive Sweeteners: Current Use and Health Perspectives". Diabetes Care. 35 (8): 1798–1808. doi:10.2337/dc12-9002.
- ^ Xu, Bocheng; Fu, Jie; Qiao, Yanxiang; Cao, Jinping; Deehan, Edward C; Li, Zhi; Jin, Mingliang; Wang, Xinxia; Wang, Yizhen (1 June 2021). "Higher intake of microbiota-accessible carbohydrates and improved cardiometabolic risk factors: a meta-analysis and umbrella review of dietary management in patients with type 2 diabetes". The American Journal of Clinical Nutrition. 113 (6): 1515–1530. doi:10.1093/ajcn/nqaa435.
- ^ Giosuè, Annalisa; Calabrese, Ilaria; Riccardi, Gabriele; Vaccaro, Olga; Vitale, Marilena (September 2022). "Consumption of different animal-based foods and risk of type 2 diabetes: An umbrella review of meta-analyses of prospective studies". Diabetes Research and Clinical Practice. 191: 110071. doi:10.1016/j.diabres.2022.110071.
- ^ Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF (July 2016). "Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes: A Meta-analysis". JAMA. 316 (3): 313–24. doi:10.1001/jama.2016.9400. PMID 27434443.
- ^ Boussageon R, Supper I, Bejan-Angoulvant T, Kellou N, Cucherat M, Boissel JP, Kassai B, Moreau A, Gueyffier F, Cornu C (2012). Groop L (ed.). "Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials". PLOS Medicine. 9 (4): e1001204. doi:10.1371/journal.pmed.1001204. PMC 3323508. PMID 22509138.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, Meeran K (April 2018). "Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis". JAMA. 319 (15): 1580–1591. doi:10.1001/jama.2018.3024. PMC 5933330. PMID 29677303.
- ^ Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH (July 2007). Richter B (ed.). "Rosiglitazone for type 2 diabetes mellitus" (PDF). The Cochrane Database of Systematic Reviews (3): CD006063. doi:10.1002/14651858.CD006063.pub2. PMC 7389529. PMID 17636824.
- ^ Chen X, Yang L, Zhai SD (December 2012). "Risk of cardiovascular disease and all-cause mortality among diabetic patients prescribed rosiglitazone or pioglitazone: a meta-analysis of retrospective cohort studies". Chinese Medical Journal. 125 (23): 4301–6. PMID 23217404.
- ^ a b Ipsen EØ, Madsen KS, Chi Y, Pedersen-Bjergaard U, Richter B, Metzendorf MI, Hemmingsen B, et al. (Cochrane Metabolic and Endocrine Disorders Group) (November 2020). "Pioglitazone for prevention or delay of type 2 diabetes mellitus and its associated complications in people at risk for the development of type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews. 2020 (11): CD013516. doi:10.1002/14651858.CD013516.pub2. PMC 8092670. PMID 33210751.
- ^ Swinnen SG, Simon AC, Holleman F, Hoekstra JB, Devries JH (July 2011). Simon AC (ed.). "Insulin detemir versus insulin glargine for type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews (7): CD006383. doi:10.1002/14651858.CD006383.pub2. PMC 6486036. PMID 21735405.
- ^ Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B, Philip S (July 2010). "Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation". Health Technology Assessment. 14 (36): 1–248. doi:10.3310/hta14360. PMID 20646668.
- ^ Mitchell S, Malanda B, Damasceno A, Eckel RH, Gaita D, Kotseva K, et al. (September 2019). "A Roadmap on the Prevention of Cardiovascular Disease Among People Living With Diabetes". Global Heart. 14 (3): 215–240. doi:10.1016/j.gheart.2019.07.009. PMID 31451236.
- ^ Brunström M, Carlberg B (February 2016). "Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses". BMJ. 352: i717. doi:10.1136/bmj.i717. PMC 4770818. PMID 26920333.
- ^ Brunström M, Carlberg B (September 2019). "Benefits and harms of lower blood pressure treatment targets: systematic review and meta-analysis of randomised placebo-controlled trials". BMJ Open. 9 (9): e026686. doi:10.1136/bmjopen-2018-026686. PMC 6773352. PMID 31575567.
- ^ Cheng J, Zhang W, Zhang X, Han F, Li X, He X, et al. (May 2014). "Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis". JAMA Internal Medicine. 174 (5): 773–785. doi:10.1001/jamainternmed.2014.348. PMID 24687000.
- ^ Zheng SL, Roddick AJ, Ayis S (September 2017). "Effects of aliskiren on mortality, cardiovascular outcomes and adverse events in patients with diabetes and cardiovascular disease or risk: A systematic review and meta-analysis of 13,395 patients". Diabetes & Vascular Disease Research. 14 (5): 400–406. doi:10.1177/1479164117715854. PMC 5600262. PMID 28844155.
- ^ a b Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, Rosano GM, Davis BR, Ridao M, et al. (March 2016). "Cardiovascular and Renal Outcomes of Renin-Angiotensin System Blockade in Adult Patients with Diabetes Mellitus: A Systematic Review with Network Meta-Analyses". PLOS Medicine. 13 (3): e1001971. doi:10.1371/journal.pmed.1001971. PMC 4783064. PMID 26954482.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, Rosenson RS, Williams CD, Wilson PW, Kirkman MS (June 2010). "Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation". Diabetes Care. 33 (6): 1395–402. doi:10.2337/dc10-0555. PMC 2875463. PMID 20508233.
- ^ Mirhosseini N, Vatanparast H, Mazidi M, Kimball SM (September 2017). "The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis". The Journal of Clinical Endocrinology and Metabolism. 102 (9): 3097–3110. doi:10.1210/jc.2017-01024. PMID 28957454.
- ^ Neves AL, Freise L, Laranjo L, Carter AW, Darzi A, Mayer E (December 2020). "Impact of providing patients access to electronic health records on quality and safety of care: a systematic review and meta-analysis". BMJ Quality & Safety. 29 (12): 1019–1032. doi:10.1136/bmjqs-2019-010581. PMC 7785164. PMID 32532814.
- ^ "Sharing electronic records with patients led to improved control of type two diabetes". NIHR Evidence (Plain English summary). 2020-10-21. doi:10.3310/alert_42103.
- ^ Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ (September 2009). "The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation". Health Technology Assessment. 13 (41): 1–190, 215–357, iii–iv. doi:10.3310/hta13410. hdl:10536/DRO/DU:30064294. PMID 19726018.
- ^ Frachetti KJ, Goldfine AB (April 2009). "Bariatric surgery for diabetes management". Current Opinion in Endocrinology, Diabetes and Obesity. 16 (2): 119–24. doi:10.1097/MED.0b013e32832912e7. PMID 19276974. S2CID 31797748.
- ^ a b Schulman AP, del Genio F, Sinha N, Rubino F (September–October 2009). ""Metabolic" surgery for treatment of type 2 diabetes mellitus". Endocrine Practice. 15 (6): 624–31. doi:10.4158/EP09170.RAR. PMID 19625245.
- ^ Colucci RA (January 2011). "Bariatric surgery in patients with type 2 diabetes: a viable option". Postgraduate Medicine. 123 (1): 24–33. doi:10.3810/pgm.2011.01.2242. PMID 21293081. S2CID 207551737.
- ^ Dixon JB, le Roux CW, Rubino F, Zimmet P (June 2012). "Bariatric surgery for type 2 diabetes". Lancet. 379 (9833): 2300–11. doi:10.1016/S0140-6736(12)60401-2. PMID 22683132. S2CID 5198462.
- ^ Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, Amiel SA, Kaplan LM, Taroncher-Oldenburg G, Cummings DE (June 2016). "Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations". Diabetes Care. 39 (6): 861–77. doi:10.2337/dc16-0236. PMID 27222544.
- ^ International Diabetes Federation 2021, p. 33.
- ^ Kahn, Ferris & O'Neill 2020, Epidemiology.
- ^ Abate N, Chandalia M (2001). "Ethnicity and type 2 diabetes: focus on Asian Indians". Journal of Diabetes and Its Complications. 15 (6): 320–7. doi:10.1016/S1056-8727(01)00161-1. PMID 11711326.
- ^ Carulli L, Rondinella S, Lombardini S, Canedi I, Loria P, Carulli N (November 2005). "Review article: diabetes, genetics and ethnicity". Alimentary Pharmacology & Therapeutics. 22 (Suppl 2): 16–9. doi:10.1111/j.1365-2036.2005.02588.x. PMID 16225465. S2CID 35041696.
- ^ Wild S, Roglic G, Green A, Sicree R, King H (May 2004). "Global prevalence of diabetes: estimates for the year 2000 and projections for 2030". Diabetes Care. 27 (5): 1047–53. doi:10.2337/diacare.27.5.1047. PMID 15111519.
- ^ a b c d e f g h Zajac J, Shrestha A, Patel P, Poretsky L (2009). "The Main Events in the History of Diabetes Mellitus". In Poretsky L (ed.). Principles of diabetes mellitus (2nd ed.). New York: Springer. pp. 3–16. ISBN 978-0-387-09840-1. OCLC 663097550.
- ^ a b Koutroumpakis E, Jozwik B, Aguilar D, Taegtmeyer H (March 2020). "Strategies of Unloading the Failing Heart from Metabolic Stress". The American Journal of Medicine (Review). 133 (3): 290–296. doi:10.1016/j.amjmed.2019.08.035. PMC 7054139. PMID 31520618.
- ^ "New tool for assessing the severity of type 2 diabetes could help personalise treatment and improve outcomes". NIHR Evidence (Plain English summary). National Institute for Health and Care Research. 2020-08-07. doi:10.3310/alert_40652.
- ^ Zghebi SS, Mamas MA, Ashcroft DM, Salisbury C, Mallen CD, Chew-Graham CA, et al. (May 2020). "Development and validation of the DIabetes Severity SCOre (DISSCO) in 139 626 individuals with type 2 diabetes: a retrospective cohort study". BMJ Open Diabetes Research & Care. 8 (1): e000962. doi:10.1136/bmjdrc-2019-000962. PMC 7228474. PMID 32385076.
Works cited
- Kahn CR, Ferris HA, O'Neill BT (2020). "Pathophysiology of Type 1 Diabetes Mellitus". Williams Textbook of Endocrinology (14 ed.). Elsevier. pp. 1349–1370.
- International Diabetes Federation (2021). IDF Diabetes Atlas (PDF) (10 ed.). ISBN 9782930229980. Retrieved 18 March 2022.
