Symbiogenesis: Difference between revisions
Restore fixed image |
→Secondary endosymbiosis: citation details |
||
| Line 43: | Line 43: | ||
Despite the diversity of organisms containing plastids, the morphology, biochemistry, genomic organisation, and molecular phylogeny of plastid RNAs and proteins suggest a single origin of all extant plastids – although this theory is still debated.<ref>{{cite journal |author=McFadden GI, van Dooren GG |title=Evolution: red algal genome affirms a common origin of all plastids |journal=Curr. Biol. |volume=14 |issue=13 |pages=R514–6 |year=2004 |month=July |pmid=15242632 |doi=10.1016/j.cub.2004.06.041 |url=http://linkinghub.elsevier.com/retrieve/pii/S0960982204004464}}</ref><ref>{{cite journal |author=Gould SB, Waller RF, McFadden GI |title=Plastid evolution |journal=Annu Rev Plant Biol |volume=59 |issue= 1|pages=491–517 |year=2008 |pmid=18315522 |doi=10.1146/annurev.arplant.59.032607.092915 |url=http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.arplant.59.032607.092915?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov}}</ref> |
Despite the diversity of organisms containing plastids, the morphology, biochemistry, genomic organisation, and molecular phylogeny of plastid RNAs and proteins suggest a single origin of all extant plastids – although this theory is still debated.<ref>{{cite journal |author=McFadden GI, van Dooren GG |title=Evolution: red algal genome affirms a common origin of all plastids |journal=Curr. Biol. |volume=14 |issue=13 |pages=R514–6 |year=2004 |month=July |pmid=15242632 |doi=10.1016/j.cub.2004.06.041 |url=http://linkinghub.elsevier.com/retrieve/pii/S0960982204004464}}</ref><ref>{{cite journal |author=Gould SB, Waller RF, McFadden GI |title=Plastid evolution |journal=Annu Rev Plant Biol |volume=59 |issue= 1|pages=491–517 |year=2008 |pmid=18315522 |doi=10.1146/annurev.arplant.59.032607.092915 |url=http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.arplant.59.032607.092915?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov}}</ref> |
||
Some species including ''[[Pediculus humanus]]'' have multiple chromosomes in the mitochondrion. This and the phylogenetics of the genes encoded within the mitochondrion suggests that the ancestors of mitochondria may have been acquired on several occasions rather than just once.<ref>Georgiades K |
Some species including ''[[Pediculus humanus]]'' have multiple chromosomes in the mitochondrion. This and the phylogenetics of the genes encoded within the mitochondrion suggests that the ancestors of mitochondria may have been acquired on several occasions rather than just once.<ref>{{cite journal|author=Georgiades, K. and Raoult, D.|year=2011|title=The rhizome of ''Reclinomonas americana'', ''Homo sapiens'', ''Pediculus humanus'' and ''Saccharomyces cerevisiae'' mitochondria|url=http://www.biology-direct.com/content/6/1/55|journal=Biology Direct|volume=6|issue=55}}</ref> |
||
==See also== |
==See also== |
||
Revision as of 16:40, 7 April 2013

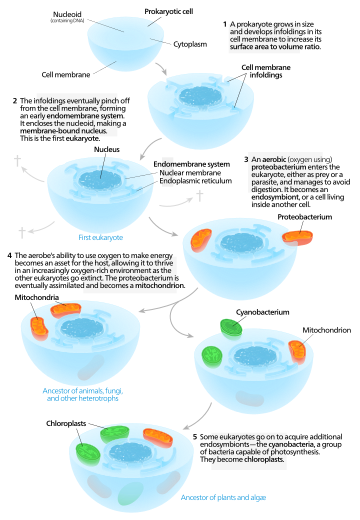
The endosymbiotic theory postulates that several key organelles of eukaryotes originated as symbioses between separate single-celled organisms. According to this theory, mitochondria and plastids (e.g. chloroplasts)--and possibly other organelles--represent formerly free-living bacteria that were taken inside another cell as an endosymbiont. Molecular and biochemical evidence suggest the mitochondrion developed from proteobacteria (in particular, Rickettsiales, the SAR11 clade,[1][2] or close relatives) and the chloroplast from cyanobacteria.
History

The endosymbiotic (Greek: ἔνδον endon "within", σύν syn "together" and βίωσις biosis "living") theories were first articulated by the Russian botanist Konstantin Mereschkowski in 1910,[3] although the fundamental elements of the theory were described in a paper five years earlier.[4][5] Mereschkowski was familiar with work by botanist Andreas Schimper, who had observed in 1883 that the division of chloroplasts in green plants closely resembled that of free-living cyanobacteria, and who had himself tentatively proposed (in a footnote) that green plants had arisen from a symbiotic union of two organisms.[6] Ivan Wallin extended the idea of an endosymbiotic origin to mitochondria in the 1920s.[7][8] These theories were initially dismissed or ignored. More detailed electron microscopic comparisons between cyanobacteria and chloroplasts (for example studies by Hans Ris[9]), combined with the discovery that plastids and mitochondria contain their own DNA[10] (which by that stage was recognized to be the hereditary material of organisms) led to a resurrection of the idea in the 1960s.
The endosymbiotic theory was advanced and substantiated with microbiological evidence by Lynn Margulis in a 1967 paper, The Origin of Mitosing Eukaryotic Cells.[11] In her 1981 work Symbiosis in Cell Evolution she argued that eukaryotic cells originated as communities of interacting entities, including endosymbiotic spirochaetes that developed into eukaryotic flagella and cilia. This last idea has not received much acceptance, because flagella lack DNA and do not show ultrastructural similarities to bacteria or archaea. See also Evolution of flagella. According to Margulis and Dorion Sagan,[12] "Life did not take over the globe by combat, but by networking" (i.e., by cooperation). The possibility that peroxisomes may have an endosymbiotic origin has also been considered, although they lack DNA. Christian de Duve proposed that they may have been the first endosymbionts, allowing cells to withstand growing amounts of free molecular oxygen in the Earth's atmosphere. However, it now appears that they may be formed de novo, contradicting the idea that they have a symbiotic origin.[13]
It is thought that over millennia these endosymbionts transferred some of their own DNA to the host cell's nucleus during the evolutionary transition from a symbiotic community to an instituted eukaryotic cell (called "serial endosymbiosis"). The endosymbiotic theory is considered to be a type of saltational evolution.[14]
From endosymbionts to organelles
According to Keeling and Archibald,[15] the usual way to distinguish organelles from endosymbionts is by their reduced genome sizes. As an endosymbiont evolves into an organelle, most of their genes are transferred to the host cell genome. The host cell and organelle need to develop a transport mechanism that enables transfer back of the protein products needed by the organelle but now manufactured by the cell. However, using the example of the freshwater amoeboid Paulinella chromatophora, which contains chromatophores found to be evolved from cyanobacteria, these authors argue that this is not the only possible criterion, another one being that the host cell has assumed control of the regulation of the former endosymbiont's division, bringing it in synchrony with the cell's own division.[15] Nowack and his colleagues[16] performed gene sequencing on the chromatophore (1.02Mb) and found that only 867 proteins were encoded by these photosynthetic cells. Comparisons with their closest free living cyanobacteria of the genus Synechococcus (having a genome size of 3Mb with 3300 genes) revealed that chromatophores underwent a drastic genome shrinkage. Chromatophores contained genes that were accountable for photosynthesis but were deficient in genes that could carry out other biosynthetic functions signifying that these endosymbiotic cells were highly dependent on their hosts for their survival and growth mechanisms. Thus, these chromatophores were found to be non-functional for organelle-specific purposes when compared to mitochondria and plastids. This distinction could have promoted the early evolution of photosynthetic organelles.
Evidence
Evidence that mitochondria and plastids arose from bacteria is as follows:[17][18][19]
- New mitochondria and plastids are formed only through a process similar to binary fission.
- In some algae, such as Euglena, the plastids can be destroyed by certain chemicals or prolonged absence of light without otherwise affecting the cell. In such a case, the plastids will not regenerate. This shows that the plastid regeneration relies on an extracellular source, such as from cell division or endosymbiosis.
- They are surrounded by two or more membranes, and the innermost of these shows differences in composition from the other membranes of the cell.
- Both mitochondria and plastids contain DNA that is different from that of the cell nucleus and that is similar to that of bacteria (both in their size and circular form).
- DNA sequence analysis and phylogenetic estimates suggest that nuclear DNA contains genes that probably came from plastids.
- These organelles' ribosomes are like those found in bacteria (70S).
- Proteins of organelle origin, like those of bacteria, use N-formylmethionine as the initiating amino acid.
- Much of the internal structure and biochemistry of plastids, for instance the presence of thylakoids and particular chlorophylls, is very similar to that of cyanobacteria. Phylogenetic estimates constructed with bacteria, plastids, and eukaryotic genomes also suggest that plastids are most closely related to cyanobacteria.
- Mitochondria have several enzymes and transport systems similar to those of bacteria.
- Some proteins encoded in the nucleus are transported to the organelle, and both mitochondria and plastids have small genomes compared to bacteria. This is consistent with an increased dependence on the eukaryotic host after forming an endosymbiosis. Most genes on the organellar genomes have been lost or moved to the nucleus. Most genes needed for mitochondrial and plastid function are located in the nucleus. Many originate from the bacterial endosymbiont.
- Plastids are present in very different groups of protists, some of which are closely related to forms lacking plastids. This suggests that if chloroplasts originated de novo, they did so multiple times, in which case their close similarity to each other is difficult to explain.
- Many of these protists contain "primary" plastids that have not yet been acquired from other plastid-containing eukaryotes.
- Among eukaryotes that acquired their plastids directly from bacteria (known as Archaeplastida), the glaucophyte algae have chloroplasts that strongly resemble cyanobacteria. In particular, they have a peptidoglycan cell wall between the two membranes.
Secondary endosymbiosis
Primary endosymbiosis involves the engulfment of a bacterium by another free living organism. Secondary endosymbiosis occurs when the product of primary endosymbiosis is itself engulfed and retained by another free living eukaryote. Secondary endosymbiosis has occurred several times and has given rise to extremely diverse groups of algae and other eukaryotes. Some organisms can take opportunistic advantage of a similar process, where they engulf an alga and use the products of its photosynthesis, but once the prey item dies (or is lost) the host returns to a free living state. Obligate secondary endosymbionts become dependent on their organelles and are unable to survive in their absence (for a review see McFadden 2001[20]). RedToL, the Red Algal Tree of Life Initiative funded by the National Science Foundation highlights the role red algae or Rhodophyta played in the evolution of our planet through secondary endosymbiosis.
One possible secondary endosymbiosis in process has been observed by Okamoto & Inouye (2005). The heterotrophic protist Hatena behaves like a predator until it ingests a green alga, which loses its flagella and cytoskeleton, while Hatena, now a host, switches to photosynthetic nutrition, gains the ability to move towards light and loses its feeding apparatus.
The process of secondary endosymbiosis left its evolutionary signature within the unique topography of plastid membranes. Secondary plastids are surrounded by three (in euglenophytes and some dinoflagellates) or four membranes (in haptophytes, heterokonts, cryptophytes, and chlorarachniophytes). The two additional membranes are thought to correspond to the plasma membrane of the engulfed alga and the phagosomal membrane of the host cell. The endosymbiotic acquisition of a eukaryote cell is represented in the cryptophytes; where the remnant nucleus of the red algal symbiont (the nucleomorph) is present between the two inner and two outer plastid membranes.[citation needed]
Despite the diversity of organisms containing plastids, the morphology, biochemistry, genomic organisation, and molecular phylogeny of plastid RNAs and proteins suggest a single origin of all extant plastids – although this theory is still debated.[21][22]
Some species including Pediculus humanus have multiple chromosomes in the mitochondrion. This and the phylogenetics of the genes encoded within the mitochondrion suggests that the ancestors of mitochondria may have been acquired on several occasions rather than just once.[23]
See also
- Anagenesis
- Hatena
- Hydrogen hypothesis
- James A. Lake
- Lichen
- Numt
- Parasite_Eve
- Protobiont
- Symbiogenesis
- Transfer of mitochondrial and chloroplast DNA to the nucleus
- Viral eukaryogenesis (hypothesis that the cell nucleus originated from endosymbiosis).
Notes
- ^ "Mitochondria Share an Ancestor With SAR11, a Globally Significant Marine Microbe". ScienceDaily. July 25, 2011. Retrieved 2011-07-26.
- ^ J. Cameron Thrash; et al. (2011). "Phylogenomic evidence for a common ancestor of mitochondria and the SAR11 clade". Scientific Reports. doi:10.1038/srep00013.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Mereschkowsky, Konstantin (1910). "Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Ent‐stehung der Organismen". Biol Centralbl. 30: 353‐367.
- ^ Mereschkowski C (1905). "Über Natur und Ursprung der Chromatophoren im Pflanzenreiche". Biol Centralbl. 25: 593–604.
- ^ Martin, William. "Modern endosymbiotic theory: Getting lateral gene transfer in-to the equation". Journal of Endocytobiosis and Cell Research. 23: 1–5.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Schimper AFW (1883). "Über die Entwicklung der Chlorophyllkörner und Farbkörper". Bot. Zeitung. 41: 105–14, 121–31, 137–46, 153–62.
- ^ Wallin IE (1923). "The Mitochondria Problem". The American Naturalist. 57 (650): 255–61. doi:10.1086/279919.
- ^ Wallin, I.E. (1927). Symbionticism and the origin of species. Baltimore: Williams & Wilkins Company. p. 171.
- ^ Ris H, Singh RN (1961). "Electron microscope studies on blue-green algae". J Biophys Biochem Cytol. 9 (1): 63–80. doi:10.1083/jcb.9.1.63. PMC 2224983. PMID 13741827.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Stocking C and Gifford E (1959). "Incorporation of thymidine into chloroplasts of Spirogyra". Biochem. Biophys. Res. Comm. 1 (3): 159–64. doi:10.1016/0006-291X(59)90010-5.
- ^ Lynn Sagan (1967). "On the origin of mitosing cells". J Theor Bio. 14 (3): 255–274. doi:10.1016/0022-5193(67)90079-3. PMID 11541392.
- ^ Margulis, Lynn (2001). "Marvellous microbes". Resurgence. 206: 10–12.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gabaldón T, Snel B, van Zimmeren F, Hemrika W, Tabak H, Huynen MA (2006). "Origin and evolution of the peroxisomal proteome". Biol. Direct. 1 (1): 8. doi:10.1186/1745-6150-1-8. PMC 1472686. PMID 16556314.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) (Provides evidence that contradicts an endosymbiotic origin of peroxisomes. Instead it is suggested that they evolutionarily originate from the Endoplasmic Reticulum) - ^ Michael Syvanen, Clarence I. Kado Horizontal Gene Transfer Academic Press, p. 405 ISBN 978-0126801262
- ^ a b Keeling, P. J. (2008). "Organelle evolution: what's in a name?". Current Biology. 18: 345–347. doi:10.1016/j.cub.2008.02.065. PMID 18430636.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Nowack, E.C. (2008). "Chromatophore genome sequence of Paulinella sheds light on acquisition of photosynthesis by eukaryotes". Current Biology. 18: 410–418. doi:10.1016/j.cub.2008.02.051. PMID 18356055.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ [1] Kimball, J. 2010. Kimball's Biology Pages. Accessed October 13, 2010. An online open source biology text by Harvard professor, and author of a general biology text, John W. Kimball.
- ^ Reece, J., Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson, 2010. Campbell Biology. 9th Edition Benjamin Cummings; 9th Ed. (October 7, 2010)
- ^ Raven, P., George Johnson, Kenneth Mason, Jonathan Losos, Susan Singer, 2010. Biology. McGraw-Hill 9th Ed. (January 14, 2010)
- ^ McFadden GI (2001). "Primary and secondary endosymbiosis and the origin of plastids". J Phycology. 37 (6): 951–9. doi:10.1046/j.1529-8817.2001.01126.x.
- ^ McFadden GI, van Dooren GG (2004). "Evolution: red algal genome affirms a common origin of all plastids". Curr. Biol. 14 (13): R514–6. doi:10.1016/j.cub.2004.06.041. PMID 15242632.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Gould SB, Waller RF, McFadden GI (2008). "Plastid evolution". Annu Rev Plant Biol. 59 (1): 491–517. doi:10.1146/annurev.arplant.59.032607.092915. PMID 18315522.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Georgiades, K. and Raoult, D. (2011). "The rhizome of Reclinomonas americana, Homo sapiens, Pediculus humanus and Saccharomyces cerevisiae mitochondria". Biology Direct. 6 (55).
{{cite journal}}: CS1 maint: multiple names: authors list (link)
References
- Alberts, Bruce (2002). Molecular biology of the cell. New York: Garland Science. ISBN 0-8153-3218-1. (General textbook)
- Blanchard JL, Lynch M (2000). "Organellar genes: why do they end up in the nucleus?". Trends Genet. 16 (7): 315–20. doi:10.1016/S0168-9525(00)02053-9. PMID 10858662.
{{cite journal}}: Unknown parameter|month=ignored (help) (Discusses theories on how mitochondria and chloroplast genes are transferred into the nucleus, and also what steps a gene needs to go through in order to complete this process.) - Jarvis P (2001). "Intracellular signalling: the chloroplast talks!". Curr. Biol. 11 (8): R307–10. doi:10.1016/S0960-9822(01)00171-3. PMID 11369220.
{{cite journal}}: Unknown parameter|month=ignored (help) (Recounts evidence that chloroplast-encoded proteins affect transcription of nuclear genes, as opposed to the more well-documented cases of nuclear-encoded proteins that affect mitochondria or chloroplasts.) - Brinkman FS; Blanchard JL; Cherkasov A; et al. (2002). "Evidence that plant-like genes in Chlamydia species reflect an ancestral relationship between Chlamydiaceae, cyanobacteria, and the chloroplast". Genome Res. 12 (8): 1159–67. doi:10.1101/gr.341802. PMC 186644. PMID 12176923.
{{cite journal}}: Explicit use of et al. in:|author3=(help); Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - Okamoto N, Inouye I (2005). "A secondary symbiosis in progress?". Science. 310 (5746): 287. doi:10.1126/science.1116125. PMID 16224014.
{{cite journal}}: Unknown parameter|month=ignored (help) - Cohen WD, Gardner RS (1959). "Viral Theory and Endosymbiosis" (PDF). (Discusses theory of origin of eukaryotic cells by incorporating mitochondria and chloroplasts into anaerobic cells with emphasis on 'phage bacterial and putative viral mitochondrial/chloroplast interactions.)