Helminthiasis: Difference between revisions
I think it is useful to highlight hookworm infection under see also as it contains quite a bit of complimentary information. I know it is already linked in the article but not in a very prominent spot. |
→Deaths: GBD 2013 |
||
| Line 168: | Line 168: | ||
Estimates for the annual death toll which is directly due to soil transmitted helminthiasis is as high as 135,000.<ref name="who" /><ref name=":0">{{cite journal|journal=PLoS Negl Trop Dis |year=2012|volume=6|issue=4|pages=e1582|doi=10.1371/journal.pntd.0001582|title=A research agenda for helminth diseases of humans: the problem of helminthiases|author=Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basáñez MG |pmid=22545164 |pmc=3335854 }}</ref><ref name="yap">{{cite journal|author= Yap P, Fürst T, Müller I, Kriemler S, Utzinger J, Steinmann P|year=2012 |pmc= |pmid= 22951972|title= Determining soil-transmitted helminth infection status and physical fitness of school-aged children|journal= Journal of Visualized Experiments| volume= 66 |issue= |pages= e3966|url= | doi=10.3791/3966}}</ref> The death toll due to the malnutrition link is likely to be much higher. |
Estimates for the annual death toll which is directly due to soil transmitted helminthiasis is as high as 135,000.<ref name="who" /><ref name=":0">{{cite journal|journal=PLoS Negl Trop Dis |year=2012|volume=6|issue=4|pages=e1582|doi=10.1371/journal.pntd.0001582|title=A research agenda for helminth diseases of humans: the problem of helminthiases|author=Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basáñez MG |pmid=22545164 |pmc=3335854 }}</ref><ref name="yap">{{cite journal|author= Yap P, Fürst T, Müller I, Kriemler S, Utzinger J, Steinmann P|year=2012 |pmc= |pmid= 22951972|title= Determining soil-transmitted helminth infection status and physical fitness of school-aged children|journal= Journal of Visualized Experiments| volume= 66 |issue= |pages= e3966|url= | doi=10.3791/3966}}</ref> The death toll due to the malnutrition link is likely to be much higher. |
||
The 1990-2013 [[Global Burden of Disease]] study estimated 5,500 direct deaths due to schistosomiasis,<ref>{{cite journal|last1=Naghavi, M| last2=Wang, H| last3= et al.|title=Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013|journal=The Lancet|volume=385|issue=9963|year=2015|pages=117–171|issn=01406736|doi=10.1016/S0140-6736(14)61682-2}}</ref> whilst more than 200,000 people are estimated to die yearly from causes that are related to schistosomiasis.<ref name="The2013">{{cite journal|last=Thétiot-Laurent|first=SA|author2=Boissier, J |author3=Robert, A |author4= Meunier, B |title=Schistosomiasis Chemotherapy|journal=Angewandte Chemie (International ed. in English)|date=Jun 27, 2013|pmid=23813602|volume=52|issue=31|pages=7936–56|doi=10.1002/anie.201208390}}</ref> Another 20 million have severe consequences from the disease.<ref>{{cite journal |author=Kheir MM, Eltoum IA, Saad AM, Ali MM, Baraka OZ, Homeida MM |title=Mortality due to schistosomiasis mansoni: a field study in Sudan |journal=Am. J. Trop. Med. Hyg. |volume=60 |issue=2 |pages=307–10 |date=February 1999 |pmid=10072156 |last2=Eltoum |last3=Saad |last4=Ali |last5=Baraka |last6=Homeida }}</ref> It is the most deadly of the [[neglected tropical diseases]].<ref name="CDC2011">{{cite web|title=Neglected Tropical Diseases|url=http://www.cdc.gov/globalhealth/ntd/diseases/schisto_burden.html|website=cdc.gov|accessdate=28 November 2014|date=June 6, 2011}}</ref> |
|||
{| class="wikitable" |
{| class="wikitable" |
||
Revision as of 12:04, 22 June 2015
| Helminthiasis | |
|---|---|
| Specialty | Infectious diseases |
Helminthiasis /ˌhɛlminˈθaɪəsis/ (alternatively spelled helminthosis; plural helminthiases), also known as helminth infection or worm infection, is any macroparasitic disease of humans and other animals in which a part of the body is infected with parasitic worms (helminths). These parasites are broadly classified into tapeworms, flukes, and roundworms. They often live in the gastrointestinal tract of their hosts, but may also burrow into other organs, where they induce physiological damage. They remain the major cause of wildlife diseases, economic crises in the livestock industry, and human socio-economic problems in developing countries.
Some types of helminthiases are among the neglected tropical diseases targeted under the joint action of the world's leading pharmaceutical companies and non-governmental organizations through an ambitious project called "London Declaration on Neglected Tropical Diseases" which was launched on 30 January 2012. It aims to control or eradicate those particular diseases by 2020, by ensuring necessary supply of drugs and other intervention, and promoting sanitation and health education.[1]
Soil-transmitted helminthiasis (SHT) and schistosomiasis are the most important group of helminthiases, collectively belonging to the "neglected tropical diseases".[2] Soil-transmitted helminthiases are responsible for parasitic infections in a quarter of the total human population.[3] One well-known example of soil-transmitted helminthiases is ascariasis. As of 2014, schistosomiasis is the most prevalent of all parasitic infections in humans.[4]
Signs and symptoms


Light infections
In cases of light infections there can be no symptoms.
Tissue damage and infections
Heavy infections directly damage tissues as the parasites can block internal organs or exert immense pressure in the gut. Infections are predominantly found in alimentary tract and sometimes in circulatory system, as the parasites inhabit these organs. General symptoms are stomachache, fever, vomiting, diarrhea, loss of appetite, loss of blood, fatigue, and listlessness.
Antimesenteric splitting of the outer layers of the bowel wall can occur due to large amount of helminths, such as ascaris: The bowel can be so tightly packed with ascaris that it becomes semi-rigid and inflexible and the outer layers of the bowel wall, which are the muscle layers, split under the tension.
Abdominal distension and abdominal pain might be the symptoms together with vomiting, sometimes vomiting of worms.[5] Patients might present with peritonitis or a gangrenous bowel.[5] This often requires emergency surgery. Volvulus complicating ascariasis still carries a high mortality and morbidity rate.[5] Early detection and early operative intervention is very important.[5]
Morbidity
In humans, under chronic infections, such as those in schistosomiasis, extreme morbidity is the common symptom.[6] Morbidity is accompanied by persistent poverty, decreased productivity, poor birth outcomes, poor school and work performance, and poor socioeconomic development.[7] A severe case of taeniasis can occur when the brain is infected by accidental ingestion of cysts, a clinical condition called neurocysticercosis, which is the leading cause of acquired epilepsy.[8]
Immune reactions
Indirect effects also associate with the disease. As pathogens, helminths induce immune reactions. Immune-mediated inflammatory changes occur in the skin, lung, liver, intestine, CNS, and eyes as they invade these tissues.The migration of Ascaris larvae through the respiratory passageways can also lead to temporary asthma and other respiratory symptoms.[9] Systemic changes such as eosinophilia, edema, and joint pain reflect local allergic responses to parasites.[10] In many cases, they can induce hypersensitivity leading to an acute allergy reaction called anaphylaxis. These immune responses can lead to increased susceptibility to other infections such as tuberculosis, HIV and malaria.[11][12] Coinfection cases have become serious medical problems particularly in African countries.[13] Heavy infection reduces HIV progression and viral load, most likely by improving helminth-induced immune suppression.[14]
Malnutrition
Helminthiasis is associated with nutritional problems such as vitamin deficiencies, stunting, anemia, and protein-energy malnutrition, which in turn affect cognitive ability and intellectual development.[15] This relationship is particularly alarming because it is gradual and often relatively asymptomatic.[16] Worms most probably compete directly with their hosts for nutrients; but the magnitude of this effect is likely to be minimal as the nutritional requirements of worms is relatively small.[17][18][19] In pigs and humans, Ascaris has been tied to temporarily induced lactose intolerance and vitamin A, amino acid, and fat malabsorption.[15] Impaired nutrient uptake may result from direct damage to the intestines' mucosal walls as a result of the worms’ presence, but it may also be a consequence of more nuanced changes, such as chemical imbalances caused by the body’s reaction to the helminths.[20] Alternatively, the worms’ release of protease inhibitors to defend against the body’s digestive process may impair the breakdown of other nutritious substances, as well.[17][19] In some cases diarrhea due to worms can cause speed “transit time” through the intestinal system, reducing absorption of nutrient.[15]
Malnutrition due to worms can give rise to anorexia.[18] A study of 459 children in Zanzibar revealed spontaneous increases in appetite after deworming.[21] This could be due to a side effect of body’s immune response to the worm and the stress of combating infection.[19] Specifically, some of the cytokines released in the immune response have been linked to anorexic reactions in animals.[17]
Helminths may also cause iron-deficiency anemia. This is most severe in heavy hookworm infections, as N. americanus and A. duodenale feed directly on the blood of their hosts. Although the daily consumption of an individual worm (0.02-0.07 ml and 0.14-0.26 ml respectively) is quite low, the collective consumption under heavy infection can be significantly high.[15] One scholar estimated that “the blood loss caused by hookworm was equivalent to the daily exsanguination of 1.5 million people”.[19] Whipworm is also attributed to anemia in the small intestine.[15][19]
Cognitive ability
Malnutrition due to helminths may directly affect cognition. This includeds low educational performances, decreased ability to focus, difficulty with abstract cognitive tasks, and “lower scores...on tests of mental and motor development...[as well as] increased fearfulness, inattentiveness, and decreased social responsiveness” among very young children.[17] Anemia has also been associated with reduced stamina for physical labor, a decline in the ability to learn new information, and “apathy, irritability, and fatigue”.[15] Study on deworming of 47 students from the Democratic Republic of the Congo, using iron supplements, shiowed that it produced better effects on mental cognition.[22] Among 159 Jamaican schoolchildren, deworming led to better “auditory short-term memory” and “scanning and retrieval of long-term memory;” which they achieved in nine-week period.[23] Studies in the Philippines and Indonesia found significant negative impacts of helminthic infection on memory and fluency,[24] and between worm infection and intellectual performance, particularly because their findings were significant in aspects of intellect that went beyond mere cognition and reaction time.[25]
School performance
Helminthiasis is attributed to absenteeism, under-enrollment, and attrition in school children.”[17]
Causes
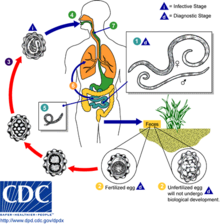

Types
Helminthiases are classified as follows (the disease names end with "-sis" and the causative worms are in brackets):
- Roundworm infection (nematodiasis)
- Filariasis (Wuchereria bancrofti, Brugia malayi infection)
- Onchocerciasis (Onchocerca volvulus infection)
- Soil-transmitted helminthiasis - this includes ascariasis (Ascaris lumbricoides infection, trichuriasis (Trichuris infection), and hookworm infection (includes Necatoriasis and Ancylostoma duodenale infection)
- Trichostrongyliasis (Trichostrongylus spp. infection)
- Dracunculiasis (guinea worm infection)
- Tapeworm infection (cestodiasis)
- Echinococcosis (Echinococcus infection)
- Hymenolepiasis (Hymenolepis infection)
- Taeniasis/cysticercosis (Taenia infection)
- Coenurosis (T. multiceps, T. serialis, T. glomerata and T. brauni infection)
- Trematode infection (trematodiasis)
- Amphistomiasis (amphistomes infection)
- Clonorchiasis (Clonorchis sinensis infection)
- Fascioliasis (Fasciola infection)
- Fasciolopsiasis (Fasciolopsis buski infection)
- Opisthorchiasis (Opisthorchis infection)
- Paragonimiasis (Paragonimus infection)
- Schistosomiasis/bilharziasis (Schistosoma infection)
- Acanthocephala infection
- Moniliformis infection
Neglected tropical diseases
Among all helminthiases, the following helminth infections are classified under neglected tropical diseases:[2][26]
- All soil-transmitted helminthiases
- Roundworm infections such as lymphatic filariasis, dracunculiasis and onchocerciasis
- Trematode infections such as schistosomiasis and food-borne trematodiases (including fascioliasis, clonorchiasis, opisthorchiasis, and paragonimiasis)
- Tapeworm infections such as cysticercosis, taeniasis, and echinococcosis
Transmission
Helminths are transmitted to the final host in several ways. The most common infection is through ingestion of contaminated vegetables, drinking water and raw or undercooked meat. Contaminated food may contain eggs of nematodes such as Ascaris, Enterobius, and Trichuris; cestodes such as Taenia, Hymenolepis, and Echinococcus; and treamtodes such as Fasciola. Raw or undercooked meats are the major sources of Taenia (pork, beef and venison), Trichinella (pork and bear), Diphyllobothrium (fish), Clonorchis (fish), and Paragonimus (crustaceans). Schistosomes and nematodes such as hookworms (Ancylostoma and Necator) and Strongyloides can directly penetrate the skin. Finally, Wuchereria, Onchocerca, and Dracunculus are transmitted by mosquitoes and flies.[6] In the developing world contaminated water is the major risk factor of infection.[27]
Infection can also take place by mistake when people eat soil on purpose - a practice called geophagy which is not uncommon in sub-Saharan Africa. The soil is eaten for example by pregnant women to counteract a real or perceived deficiency of minerals in the diet.
Most common
Of all the known helminth species, the most important helminths with respect to understanding their transmission pathways, their control, inactivation and enumeration in samples of human excreta origin, namely dried feces, faecal sludge, wastewater and sewage sludge are:[28] soil-transmitted helminths (including Ascaris lumbricoides, which is the most common worldwide, Trichuris trichiura, Necator americanus, Strongyloides stercoralis and Ancylostoma duodenale), Hymenolepis nana, Taenia saginata, Enterobius, Fasciola hepatica, Schistosoma mansoni, Toxocara canis and Toxocara cati.
Mechanism
Response to worm infection in humans is a Th2 response in the majority of cases. Inflammation of the gut may also occur, resulting in cyst-like structures forming around the egg deposits throughout the body. The host's lymphatic system is also increasingly taxed the longer helminths propagate, as they excrete toxins after feeding. These toxins are released into the intestines to be absorbed by the host's bloodstream. This phenomenon makes the host susceptible to more common diseases, such as viral and bacterial infections.
Diagnosis

Diagnosis is the mainstay in the control of helminthiases. For basic diagnosis, specific helminths can be generally identified from the feces, and their eggs microscopically examined and enumerated using the fecal egg count method. This is particularly useful in veterinary investigations.[29] But it fails to identify mixed infections, and on clinical practice, the technique is highly inaccurate and unreliable, such as those for schistosomes and soil-transmitted helmiths.[30] Sophisticated tests such as serological assays, antigen tests, and molecular diagnosis are also available;[29][31] however, they are time-consuming, expensive and not always reliable.[32]
Prevention
Prevention and control measures include: 1) use of clean water for personal and domestic uses; 2) sanitation and health education such as by promoting use of latrines; 3) awareness on personal hygiene such as hand washing and washing of food; 4) avoiding the use of uncomposted human feces as fertilizer.[3] In epidemic areas, mass deworming programs of school children is an important preventive method. Simple measures can be effective including constant wearing of shoes, soaking vegetables with bleach, adequate cooking of foods, handwashing at critical times (before contact with food and after use of the toilet), reducing open defecation, deworming of pet and proper disposal of their feces.[33]
Treatment


Medications
Broad-spectrum benzimidazoles (such as albendazole and mebendazole) are recommended for treatment of intestinal roundworm and tapeworm infections; while macrocyclic lactones (such as ivermectin) are effective against adult and migrating larval stages of nematode; and praziquantel is the drug of choice for schistosomiasis, taeniasis, and most types of food-borne trematodiases. Oxamniquine is also widely used in mass deworming programmes. Pyrantel is commonly used for veterinary nematodiasis.[35][36] Artemisinins and derivatives are proving to be candidates as drugs of choice for trematodiasis.[37]
Surgery
Surgery - even emergency surgery - might be required to remove parts of the intestine if the worm infestation has reached such a high number of worms that the worms block that piece of intestines.[5][34] Patients who are heading for surgery, for example to extract worms from the biliary tree, can be pre-treated with albendazole to kill worms prior to the surgery. In the case of intestinal obstruction it is however an emergency and there is no time to do this so surgeons have no alternative than manually pulling the worms out.[citation needed]
Mass deworming of children
In regions where the disease is common, mass deworming treatments may be performed, particularly among school-age children, who are the high-risk group.[38][39] Most of these initiatives are undertaken by the World Health Organization with positive outcomes in many regions.[40][41]
Successful deworming and positive health outcomes were also achieved by the Essential Health Care Program implemented by the Philippine Department of Education in the Philippines. UNICEF has noted it as an "outstanding example of at scale action to promote children’s health and education".[42] Deworming twice a year, supplemented with washing hands daily with soap, brushing teeth daily with fluoride, is at the core of this national program. It has also been successfully implemented in Indonesia.[43]
Deworming programs can improve school attendance by 25%.[44] This may lead to a long-term increase in income, and higher literacy rates.[45]
When deworming drugs were randomly phased into schools in Kenya, rather than to individual pupils, improved school attendance and health was observed at those schools where treatment occurred. This outcome was not dependent on whether or not the specific student had received treatment, pointing towards externality benefits of school deworming programs.[44]
Although mass dewormings improves the health of an individual, outcomes such as improved cognitive ability, nutritional benefits, physical growth and performance, and learning are still in question.[46] For example:
- A 2000 review of randomised controlled trials of children showed no special benefits on weight gain and cognitive performance.[47]
- A 2007 Cochrane analysis found no significant improvements in physical growth and cognitive performance among routinely dewormed children.[48]
- An independent report in 2007 noted no benefit on cognition and school performance, with an inconclusive effect on weight gain.[49]
- A 2012 Cochrane review found no benefit on weight, blood improvement (haemoglobin content), cognition and school performance.[50]
Epidemiology
The most affected regions are tropical and subtropical areas. Highest incidences are in subsaharan Africa, central and east Asia, and the Americas.
Infection estimates
The soil-transmitted helminths (A. lumbricoides, T. trichiura, N. americanus, A. duodenale), schistosomes, and filarial worms collectively infect more than a quarter of human population at any one time, far surpassing HIV/AIDS and malaria taken together.[29][31] Schistosomiasis alone is the second most prevalent parasitic disease of all times in humans, next only to malaria.[51]
As of 2014, the World Health Organization estimates that over 1.5 billion people (a quarter of the total population) are infected with soil-transmitted helminthiases,[3] 249 million with schistosomiasis[4] (which may have even surpassed malaria at ~207 million cases in 2013),[52] 56 million people with food-borne trematodiasis (i.e. other than schistosomiasis),[53] 120 million with filariasis,[54] 50 million people with cysticercosis,[55] at least 15 million people with onchocerciasis,[56] 1 million people with echinococcosis,[57] but only 148 people with dracunculiasis thanks to a successful eradication compaign for that particular helminth.[58]
The Global Burden of Disease study estimates a 46% (59% when age standardised) reduction in Years Lived with Disability (YLD) from 1990-2013 for all intestinal/nematode infections, with a 74% (80% when age standardised) reduction in YLD from Ascariasis.[59] It is estimated that intestinal nematode infections cause 5 million Disability-adjusted life years (DALYS) to be lost, of which hookworm infections account for more than 3 million DALYS and ascaris infections more than 1 million.[60]
By one estimate, 4.5 billion people are at constant risk of STH infection.[61]
Because of their high mobility and lower standards of hygiene, school-age children are particularly vulnerable to these parasites.[62] A child in a low-economy country is estimated to harbour at least one helminth, and multi-species infections are very common.[63]
Deaths
Estimates for the annual death toll which is directly due to soil transmitted helminthiasis is as high as 135,000.[15][42][64] The death toll due to the malnutrition link is likely to be much higher.
The 1990-2013 Global Burden of Disease study estimated 5,500 direct deaths due to schistosomiasis,[65] whilst more than 200,000 people are estimated to die yearly from causes that are related to schistosomiasis.[66] Another 20 million have severe consequences from the disease.[67] It is the most deadly of the neglected tropical diseases.[68]
| Helminth genera | Common name | Infections (million per year) | Direct deaths per year | Regions where common | |
|---|---|---|---|---|---|
| Soil transmitted helminthiasis (STH) (classified as neglected tropical disease): | |||||
| Ascaris lumbricoides | Roundworm | 1000 to 1450
807 to 1,121[69] |
20,000 | Many regions of South-east Asia, Africa, and Central and South America[42][70][71][72][73][74] | |
| Trichuris trichiura | Whipworm | 500
604-795[69] |
In moist, warm, tropical regions of Asia, Africa, Central and South America, and the Caribbean islands.[71][72][73][74][75] | ||
| Ancylostoma duodenale | Hookworm | 900 to 1300
576-740 (hookworm in general)[76] |
In tropical and subtropical countries (Sub-Saharan Africa)[72][75] | ||
| Necator americanus | |||||
| Strongyloides stercoralis | Hookworm, pinworm | 50 to 100 | Thousands | In moist rainy areas of the tropics and subtropics, in some areas of southern and eastern Europe and of the United States of America[72][73] | |
| All STH together | 1500 to 2000[3] | 135,000[15][64][77] | Tropical and subtropical areas, in particular sub-Saharan Africa, the Americas, China and east Asia.[3] | ||
| Not transmitted via soil but classified as neglected tropical disease: | |||||
| Schistosoma mansoni | Blood fluke | All types of Schistosoma together: 160 to 200
(210 "affected"[78]) |
12,000[79] 150,000 deaths from renal failure[80]
200,000 indirect deaths from "causes related to" Schistosomiasis[66] |
In tropical and subtropical regions[71][72][73][74][75] | |
| Schistosoma haematobium | 112 (in Sub-Saharan Africa alone)[80] | ||||
| Echinococcus granulosus | 3[81] | Developing countries | |||
| Not transmitted via soil and not classified as neglected tropical disease: | |||||
| Toxocara canis | Dog roundworm | 50 | Many regions of South-east Asia, Africa, and Central and South America[42][70][71][72][73][74] | ||
| Taenia solium | Pork tapeworm | 50 | South America, Southeast Asia, West Africa and East Africa[71][72][73][74] | ||
| Taenia saginata | Beef tapeworm | 50
(all types of Taenia: 40 to 60[82]) |
|||
| Hymenolepis nana | Dwarf tapeworm | 100 | |||
| Hymenolepis diminuta | Rat tapeworm | ||||
| Fasciola hepatica, Fascioloides magna |
Liver fluke | 50 | Largely in southern and eastern Asia but also in central and eastern Europe[72][73] | ||
| Fasciolopsis buski | Giant intestinal fluke | ||||
| Dracunculus medinensis | Guinea worm | Nowadays negligible thanks to eradication program[83] | Formerly widespread in India, west Africa and southern Sudan[72][73] | ||
| Trichostrongylus orientalis | Roundworm | 1-3 ("several") | Rural communities in Asia[72][73] | ||
| Other | 100 | Worldwide[72][73] | |||
| Total (number of infections) | Approx. 3.5 billion | Worldwide | |||
See also
References
- ^ London Declaration (30 January 2012). "London Declaration on Neglected Tropical Diseases" (PDF). Retrieved 2013-03-26.
- ^ a b "Neglected Tropical Diseases". cdc.gov. June 6, 2011. Retrieved 28 November 2014.
- ^ a b c d e "Soil-transmitted helminth infections". Fact sheet N°366. April 2014. Retrieved 18 October 2014. Cite error: The named reference "WHO2014" was defined multiple times with different content (see the help page).
- ^ a b "Schistosomiasis". Fact sheet N°115. WHO Media centre. February 2014. Retrieved 6 December 2014.
- ^ a b c d e Madiba TE, Hadley GP. (1996). "Surgical management of worm volvulus". S Afr J Surg. 34 (1): 33–5, discussion 35-6. PMID 8629187. Retrieved 14 March 2015.
- ^ a b Baron S (1996). "87 (Helminths: Pathogenesis and Defenses by Wakelin D". Medical Microbiology (4 ed.). Galveston (TX): The University of Texas Medical Branch at Galveston. ISBN 0963117211. PMID 21413312.
- ^ WHO (2012). "Research priorities for helminth infections". World Health Organization Technical Report Series. 972 (972): 1–174. PMID 23420950.
- ^ Del Brutto OH (2012). "Neurocysticercosis: a review". The ScientificWorldJournal. 2012: 159821. doi:10.1100/2012/159821. PMC 3261519. PMID 22312322.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ John, David T. and William A. Petri, Jr. (2006). Markell and Vogue’s Medical Parasitology, 9th Edition. Saunders Elsevier Press.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Minciullo PL, Cascio A, David A, Pernice LM, Calapai G, Gangemi S (2012). "Anaphylaxis caused by helminths: review of the literature". Eur Rev Med Pharmacol Sci. 16 (11): 1513–1518. PMID 23111963.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ van Riet E, Hartgers FC, Yazdanbakhsh M (2007). "Chronic helminth infections induce immunomodulation: consequences and mechanisms". Immunobiology. 212 (6): 475–9. doi:10.1016/j.imbio.2007.03.009. PMID 17544832.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mkhize-Kwitshana ZL, Mabaso MH (2012). "Status of medical parasitology in South Africa: new challenges and missed opportunities". Trends in Parasitology. 28 (6): 217–219. doi:10.1016/j.pt.2012.03.005. PMID 22525798.
- ^ Borkow G & Bentwich Z (2000). "Eradication of helminthic infections may be essential for successful vaccination against HIV and tuberculosis". Bulletin of the World Health Organization. 78 (11). doi:10.1590/S0042-96862000001100013.
{{cite journal}}: External link in|journal= - ^ Walson JL, Herrin BR, John-Stewart G (2009). Walson, Judd (ed.). "Deworming helminth co-infected individuals for delaying HIV disease progression". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD006419.
{{cite journal}}: External link in|journal= - ^ a b c d e f g h Report of a WHO Expert Committee (1987). Prevention and Control of Intestinal Parasitic Infections. World Health Organization, Technical Report Series 749.
- ^ Del Rosso, Joy Miller and Tonia Marek (1996). Class Action: Improving School Performance in the Developing World through Better Health and Nutrition. The World Bank, Directions in Development.
- ^ a b c d e Levinger B (1992). Nutrition, Health, and Learning: Current Issues and Trends. School Nutrition and Health Network Monograph Series, #1. Please note that this estimate is less current than the Watkins and Pollitt estimate, leading Levinger to underestimate the number infected.
- ^ a b The World Bank. "World Development Report 1993: Investing in Health" (PDF).
- ^ a b c d e Watkins WE & Pollitt E (1997). "'Stupidity or Worms': Do Intestinal Worms Impair Mental Performance?" (PDF). Psychological Bulletin. 121 (2): 171–91. doi:10.1037/0033-2909.121.2.171. PMID 9100486.
- ^ Crompton, D.W.T. (1993). Human Nutrition and Parasitic Infection. Cambridge University Press.
- ^ Stoltzfus; Rebecca J.; et al. (2003). "Low Dose Daily Iron Supplementation Improves Iron Status and Appetite but Not Anemia, whereas Quarterly Antihelminthic Treatment Improves Growth, Appetite, and Anemia in Zanzibari Preschool Children". The Journal of Nutrition. 134 (2): 348–56. PMID 14747671.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Boivin, MJ & Giordiani B (1993). "Improvements in Cognitive Performance for Schoolchildren in Zaire, Africa, Following an Iron Supplement and Treatment for Intestinal Parasites". Journal of Pediatric Psychology. 18 (2): 249–264. doi:10.1093/jpepsy/18.2.249. PMID 8492277. 2.
- ^ Nokes, C.; et al. (1992). "Parasitic Helminth Infection and Cognitive Function in School Children". Proceedings of the Royal Society of London. 247 (1319): 77–81. doi:10.1098/rspb.1992.0011. PMID 1349184.
- ^ Ezeamama; Amara E.; et al. (2005). "Helminth infection and cognitive impairment among Filipino children". The American Journal of Tropical Medical Hygiene. 72 (5): 540–548. PMC 1382476. PMID 15891127.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Sakti, Hastaning; et al. (1999). "Evidence for an Association Between Hookworm Infection and Cognitive Function in Indonesian School Children". Tropical Medicine and International Health. 4 (5): 322–334. doi:10.1046/j.1365-3156.1999.00410.x. PMID 10402967.
- ^ "Fact sheets: neglected tropical diseases". World Health Organization. WHO Media Centre. Retrieved 6 December 2014.
- ^ Charity Water; et al. (2009). "Contaminated drinking water". Charity Water.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Maya, C.; Torner-Morales, F.J.; Lucario, E.S.; Hernández, E.; Jiménez, B. (2012). "Viability of six species of larval and non-larval helminth eggs for different conditions of temperature, pH and dryness". Water Research. 46 (15): 4770–4782. doi:10.1016/j.watres.2012.06.014.
- ^ a b c Crompton DWT, Savioli L (2007). Handbook of Helminthiasis for Public Health. CRC Press, Boca Raton, Florida, US. pp. 1–362. ISBN 9781420004946.
- ^ Krauth SJ, Coulibaly JT, Knopp S, Traoré M, N'Goran EK, Utzinger J (2012). "An in-depth analysis of a piece of shit: distribution of Schistosoma mansoni and hookworm eggs in human stool". PLoS Negl Trop Dis. 6 (12): e1969. doi:10.1371/journal.pntd.0001969. PMC 3527364. PMID 23285307.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basáñez MG (2012). "A research agenda for helminth diseases of humans: the problem of helminthiases". PLoS Negl Trop Dis. 6 (4): e1582. doi:10.1371/journal.pntd.0001582. PMC 3335854. PMID 22545164.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Hunt PW, Lello J (2012). "How to make DNA count: DNA-based diagnostic tools in veterinary parasitology". Veterinary Parasitology. 186 (1–2): 101–108. doi:10.1016/j.vetpar.2011.11.055. PMID 22169224.
- ^ "How to Get Rid of Hookworms". HowToGetRidOfStuff. Retrieved 17 November 2014.
- ^ a b Fincham, J., Dhansay, A. (2006). Worms in SA's children - MRC Policy Brief. Nutritional Intervention Research Unit of the South African Medical Research Council, South Africa
- ^ "Anthelmintics". Drugs.com. Retrieved 17 November 2014.
- ^ "Overview of Anthelmintics". The Merck Veterinary Manual. Merck Sharp & Dohme Corp. Retrieved 17 November 2014.
- ^ Pérez del Villar, Luis; Burguillo, Francisco J.; López-Abán, Julio; Muro, Antonio; Keiser, Jennifer (2012). "Systematic Review and Meta-Analysis of Artemisinin Based Therapies for the Treatment and Prevention of Schistosomiasis". PLoS ONE. 7 (9): e45867. doi:10.1371/journal.pone.0045867. PMC 3448694. PMID 23029285.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ WHO (2006). Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers (PDF). WHO Press, World Health Organization, Geneva, Switzerland. pp. 1–61. ISBN 9241547103.
- ^ Prichard RK, Basáñez MG, Boatin BA, McCarthy JS, García HH, Yang GJ, Sripa B, Lustigman S (2012). "A research agenda for helminth diseases of humans: intervention for control and elimination". PLoS Negl Trop Dis. 6 (4): e1549. doi:10.1371/journal.pntd.0001549. PMC 3335868. PMID 22545163.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Bundy, Donald A.P.; Walson, Judd L.; Watkins, Kristie L. (2013). "Worms, wisdom, and wealth: why deworming can make economic sense". Trends in Parasitology. 29 (3): 142–148. doi:10.1016/j.pt.2012.12.003. PMID 23332661.
- ^ Albonico, Marco; Allen, Henrietta; Chitsulo, Lester; Engels, Dirk; Gabrielli, Albis-Francesco; Savioli, Lorenzo; Brooker, Simon (2008). "Controlling Soil-Transmitted Helminthiasis in Pre-School-Age Children through Preventive Chemotherapy". PLoS Neglected Tropical Diseases. 2 (3): e126. doi:10.1371/journal.pntd.0000126. PMC 2274864. PMID 18365031.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d UNICEF (2012) Raising Even More Clean Hands: Advancing Health, Learning and Equity through WASH in Schools, Joint Call to Action Cite error: The named reference ":0" was defined multiple times with different content (see the help page).
- ^ School Community Manual - Indonesia (formerly Manual for teachers), Fit for School. GIZ Fit for School, Philippines. 2014. ISBN 978-3-95645-250-5.
- ^ a b Miguel, Edward and Michael Kremer (2004). "Worms: Identifying Impacts on Education and Health in the Presence of Treatment Externalities" (PDF). Econometrica. 72 (1): 159–217. doi:10.1111/j.1468-0262.2004.00481.x.
- ^ Bleakley, Hoyt (2007). "Disease and Development: Evidence From Hookworm Eradication in the American South". The Quarterly Journal of Economics. 122: 73–117. doi:10.1162/qjec.121.1.73. PMC 3800113. PMID 24146438.
- ^ Hawkes, N. (2013). "Deworming debunked". BMJ. 346 (jan02 1): e8558–e8558. doi:10.1136/bmj.e8558.
- ^ Dickson, R. (2000). "Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomised trials". BMJ. 320 (7251): 1697–1701. doi:10.1136/bmj.320.7251.1697. PMC 27412. PMID 10864543.
- ^ Dickson, R; Awasthi, S; Demellweek, C; Williamson, P (2007). "WITHDRAWN: Anthelmintic drugs for treating worms in children: effects on growth and cognitive performance". The Cochrane Database of Systematic Reviews (2): CD000371. doi:10.1002/14651858.CD000371.pub2. PMID 17636634.
- ^ Taylor-Robinson, DC; Jones, AP; Garner, P (17 October 2007). "Deworming drugs for treating soil-transmitted intestinal worms in children: effects on growth and school performance". The Cochrane Database of Systematic Reviews (4): CD000371. doi:10.1002/14651858.CD000371.pub3. PMID 17943740.
- ^ Taylor-Robinson, DC; Maayan, N; Soares-Weiser, K; Donegan, S; Garner, P (2012). "Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance". The Cochrane Database of Systematic Reviews. 11: CD000371. doi:10.1002/14651858.CD000371.pub5. PMID 23152203.
- ^ WHO (2013). Schistosomiasis: progress report 2001 - 2011, strategic plan 2012 - 2020. WHO Press, World Health Organization, Geneva, Switzerland. pp. 1–270. ISBN 9789241503174.
- ^ "Malaria". Fact sheet N°94. WHO Media Centre. March 2014. Retrieved 6 December 2014.
- ^ "Foodborne trematode infections". Factsheet N°368. WHO Media Centre. 2014. Retrieved 6 December 2014.
- ^ "Lymphatic filariasis". Fact sheet N°102. WHO Media centre. March 2014. Retrieved 6 December 2014.
- ^ "Taeniasis/cysticercosis". Fact sheet N°376. WHO Media Centre. May 2014. Retrieved 6 December 2014.
- ^ "Onchocerciasis". Fact sheet N°374. WHO Media Centre. March 2014. Retrieved 6 December 2014.
- ^ "Echinococcosis". Fact sheet N°377. WHO Media Centre. March 2014. Retrieved 6 December 2014.
- ^ "Dracunculiasis (guinea-worm disease)". Fact sheet N°359 (Revised). WHO Media Centre. March 2014. Retrieved 6 December 2014.
- ^ Vos, T; Barber, RM; et al. (2015). "Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". The Lancet. doi:10.1016/S0140-6736(15)60692-4. ISSN 0140-6736.
{{cite journal}}: Explicit use of et al. in:|last3=(help) - ^ de Silva, N; Hotez, PJ; et al. (2014). "The Global Burden of Disease Study 2010: Interpretation and Implications for the Neglected Tropical Diseases". PLoS Neglected Tropical Diseases. 8 (7): e2865. doi:10.1371/journal.pntd.0002865. ISSN 1935-2735.
{{cite journal}}: Explicit use of et al. in:|last3=(help)CS1 maint: unflagged free DOI (link) - ^ Velleman, Y., Pugh, I. (2013). Under-nutrition and water, sanitation and hygiene - Water, sanitation and hygiene (WASH) play a fundamental role in improving nutritional outcomes. A successful global effort to tackle under-nutrition must include WASH. WaterAid and Share, UK
- ^ Montresor; et al. (2002). "Helminth Control in School-Age Children: A Guide for Managers of Control Programs" (PDF). World Health Organization.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Bethony, Jeffrey; Brooker, Simon; Albonico, Marco; Geiger, Stefan M; Loukas, Alex; Diemert, David; Hotez, Peter J (2006). "Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm". The Lancet. 367 (9521): 1521–1532. doi:10.1016/S0140-6736(06)68653-4. PMID 16679166.
- ^ a b Yap P, Fürst T, Müller I, Kriemler S, Utzinger J, Steinmann P (2012). "Determining soil-transmitted helminth infection status and physical fitness of school-aged children". Journal of Visualized Experiments. 66: e3966. doi:10.3791/3966. PMID 22951972.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Naghavi, M; Wang, H; et al. (2015). "Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". The Lancet. 385 (9963): 117–171. doi:10.1016/S0140-6736(14)61682-2. ISSN 0140-6736.
{{cite journal}}: Explicit use of et al. in:|last3=(help) - ^ a b Thétiot-Laurent, SA; Boissier, J; Robert, A; Meunier, B (Jun 27, 2013). "Schistosomiasis Chemotherapy". Angewandte Chemie (International ed. in English). 52 (31): 7936–56. doi:10.1002/anie.201208390. PMID 23813602.
- ^ Kheir MM, Eltoum IA, Saad AM, Ali MM, Baraka OZ, Homeida MM; Eltoum; Saad; Ali; Baraka; Homeida (February 1999). "Mortality due to schistosomiasis mansoni: a field study in Sudan". Am. J. Trop. Med. Hyg. 60 (2): 307–10. PMID 10072156.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Neglected Tropical Diseases". cdc.gov. June 6, 2011. Retrieved 28 November 2014.
- ^ a b "Parasites - Soil-transmitted Helminths (STHs)". Centres for Disease Control and Prevention (CDC). 10 January 2013. Retrieved 20 December 2014.
- ^ a b B. Jiménez (2007) Helminth ova removal from wastewater for agriculture and aquaculture reuse. Water Sciences & Technology, 55(1-2): 485-493. Cite error: The named reference ":1" was defined multiple times with different content (see the help page).
- ^ a b c d e I. Navarro, B. Jiménez, E. Cifuentes and S. Lucario (2009) Application of helminth ova infection dose curve to estimate the risks associated with biosolid application on soil, Journal of Water and Health 31-44.
- ^ a b c d e f g h i j k Blanca Jiménez, Inés Navarro (2013) Wastewater Use in Agriculture: Public Health Considerations. Encyclopedia of Environmental Management. Ed., Vol. IV, Dr. Sven Erik Jorgensen (Ed.), DOI: 10.1081/E-EEM-120046689 Copyright © 2012 by Taylor & Francis. Group, New York, NY, pp 3,512.
- ^ a b c d e f g h i j UN (2003) Water for People Water for Life. The United Nations World Water Development Report, UNESCOEd, Barcelona, Spain.
- ^ a b c d e WHO (1995) WHO Model Prescribing Information: Drug Use in Parasitic Diseases. WHO, Geneva, Switzerland.
- ^ a b c WHO (2006). WHO Guidelines for the Safe Use of Wastewater, Excreta and Greywater - Volume IV: Excreta and greywater use in agriculture. World Health Organization (WHO), Geneva, Switzerland
- ^ "Parasites - Hookworm". Centers for Disease Control and Prevention (CDC). Retrieved 20 December 2014.
- ^ Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basáñez MG (2012). "A research agenda for helminth diseases of humans: the problem of helminthiases". PLoS Negl Trop Dis. 6 (4): e1582. doi:10.1371/journal.pntd.0001582. PMC 3335854. PMID 22545164.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Fenwick, A (Mar 2012). "The global burden of neglected tropical diseases". Public health. 126 (3): 233–6. doi:10.1016/j.puhe.2011.11.015. PMID 22325616.
- ^ Lozano, R; Naghavi, M; Foreman, K; Lim, S; Shibuya, K; Aboyans, V; Abraham, J; Adair, T; et al. (Dec 15, 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
- ^ a b Luke F. Pennington and Michael H. Hsieh (2014) Immune Response to Parasitic Infections, Bentham e books, Vol 2, pp. 93-124, eISBN 978-1-60805-148-9
- ^ Elisabetta Profumo, Alessandra Ludovisi, Brigitta Buttari, Maria, Angeles Gomez Morales and Rachele Riganò (2014) Immune Response to Parasitic Infections, Bentham e books, Bentham Science Publishers, Vol 2, pp. 69-91, eISBN 978-1-60805-148-9
- ^ Eckert, J. (2005). "Helminths". In Kayser, F.H., Bienz, K.A., Eckert, J., Zinkernagel, R.M. (ed.). Medical Microbiology. Stuttgart: Thieme. pp. 560–562. ISBN 9781588902450.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ "Dracunculiasis (guinea-worm disease) Fact sheet N°359 (Revised)". World Health Organization. March 2014. Retrieved 18 March 2014.
