Halobacterium noricense: Difference between revisions
took down published article |
added taxonomy, discovery, metabolism, genome, and significance |
||
| Line 1: | Line 1: | ||
{{Italic title}} |
{{Italic title}}{{Taxobox |
||
{{Taxobox |
|||
| color = lightgrey |
| color = lightgrey |
||
| name = ''Halobacterium noricense'' |
| name = ''Halobacterium noricense'' |
||
| Line 13: | Line 12: | ||
| binomial = ''Halobacterium noricense'' |
| binomial = ''Halobacterium noricense'' |
||
| binomial_authority = Gruber et al. 2005 |
| binomial_authority = Gruber et al. 2005 |
||
}} is a [[Halophile|halophilic]], rod-shaped microorganism that thrives in environments with salt levels near saturation.<ref name=":02">{{cite journal|vauthors=Fendrihan S, Legat A, Pfaffenhuemer M, Gruber C, Weidler G, Gerbl F, Stan-Lotter H|date=August 2006|title=Extremely halophilic archaea and the issue of long-term microbial survival|journal=Re/Views in Environmental Science and Bio/Technology|volume=5|issue=2-3|pages=203–218|doi=10.1007/s11157-006-0007-y|pmc=3188376|pmid=21984879}}</ref> Despite the implication of the name, '''''Halobacterium noricense''''' is a species of archaeon first isolated from a bore core of an alpine Permian salt deposit. Its type strain is A1 (DSM 15987<sup>T</sup>, ATCC BAA-852<sup>T</sup>, NCIMB 13967<sup>T</sup>).<ref name="Gruber_2004">{{cite journal | vauthors = Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse HJ, Stan-Lotter H | title = Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum | journal = Extremophiles | volume = 8 | issue = 6 | pages = 431–9 | date = December 2004 | pmid = 15290323 | doi = 10.1007/s00792-004-0403-6 }}</ref> is actually a genus of '''Halobacterium noricense''', not <sup>T</sup>. <sup>T</sup> can be isolated from environments with high salinity such as the Dead Sea and the Great Salt Lake in Utah.<ref name=":02" /> Members of the <sup>T</sup> [[Genus]] are excellent model organisms for {{Reflist}} and {{cite journal | vauthors = Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse HJ, Stan-Lotter H | title = Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum | journal = Extremophiles | volume = 8 | issue = 6 | pages = 431–9 | date = December 2004 | pmid = 15290323 | doi = 10.1007/s00792-004-0403-6 }} due to the stability of their proteins and polymerases when exposed to high temperatures.<ref name="Gruber_20042">{{cite journal|vauthors=Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse HJ, Stan-Lotter H|date=December 2004|title=Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum|journal=Extremophiles|volume=8|issue=6|pages=431–9|doi=10.1007/s00792-004-0403-6|pmid=15290323}}</ref> To be classified in the ''Halobacterium'' genus, a microorganism must exhibit a membrane composition consisting of ether-linked [[Phosphoglycerides]] and [[Glycolipid|glycolipids]].<ref name="Gruber_20042" />[[File:Halobacteria_with_scale.jpg|link=https://en.wikipedia.org/wiki/File:Halobacteria_with_scale.jpg|thumb|Typical morphology of <i>Halobacterium</i> species]]<table class="wikitable sortable mw-collapsible"><tr><th colspan="2">''Halobacterium noricense''</th></tr><tr><th colspan="2">[[Taxonomy (biology)|Scientific classification]]</th></tr><tr><td>Domain:</td><td>[[Archaea]]</td></tr><tr><td>Kingdom:</td><td>[[Euryarchaeota]]</td></tr><tr><td>Phylum:</td><td href="Category:Halophiles">[[Euryarchaeota]]</td></tr><tr><td>Class:</td><td>[[Halobacteria]]</td></tr><tr><td>Order:</td><td>[[Halobacteriales]]</td></tr><tr><td>Family:</td><td>[[Halobacteriaceae]]</td></tr><tr><td>Genus:</td><td>[[Halobacterium]]</td></tr><tr><td>Species:</td><td>'''''H. noricense'''''</td></tr><tr><th colspan="2">[[Binomial nomenclature|Binomial name]]</th></tr><tr><td colspan="2">'''''Halobacterium noricense''''' |
|||
}} |
|||
<small>Gruber et al. 2005</small> |
|||
</td></tr><tr><th colspan="2">Type Strain</th></tr><tr><th colspan="2">'''<small>DSM 15987<sup>T</sup>, ATCC BAA-852<sup>T</sup>,</small>''' |
|||
'''<small>NCIMB 13967<sup>T</sup>, NRC-1,</small>''' |
|||
'''<small>DL1, R1, CBA1132</small>''' |
|||
'''''Halobacterium noricense''''' is a species of archaeon first isolated from a bore core of an alpine Permian salt deposit. Its type strain is A1 (DSM 15987<sup>T</sup>, ATCC BAA-852<sup>T</sup>, NCIMB 13967<sup>T</sup>).<ref name="Gruber_2004">{{cite journal | vauthors = Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse HJ, Stan-Lotter H | title = Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum | journal = Extremophiles | volume = 8 | issue = 6 | pages = 431–9 | date = December 2004 | pmid = 15290323 | doi = 10.1007/s00792-004-0403-6 }}</ref> |
|||
</th></tr></table> |
|||
== Scientific classification == |
|||
This organism is a member of the ''Halobacterium'' genus and its taxonomic classification is as follows: [[Archaea]], [[Euryarchaeota]], [[Euryarchaeota]], [[Halobacteria]], [[Halobacteriales]], [[Halobacteriaceae]], [[Halobacterium]], ''Halobacterium noricense.''<ref name=":02" /> There are currently 19 known halophilic archaeal genera and 57 known species within the ''Halobacterium'' genus.<ref name="Gruber_20042" /> |
|||
==== Relatives ==== |
|||
Three reported strains [[Halobacterium salinarum|''Halobacterium'' ''salinarium'']] NRC-1, ''Halobacterium'' sp. DL1, and ''Halobacterium'' ''salinarium'' R1 were compared to ''Halobacterium noricense'' strain CBA1132.<ref name=":0">{{Cite journal|last=Ki Lim|first=Seul|last2=Kim|first2=Joon Yong|last3=Seon Song|first3=Hye|last4=Kwon|first4=Min-Sung|last5=Lee|first5=Join|last6=Jun Oh|first6=Young|last7=Nam|first7=Young-Do|last8=Seo|first8=Myung-Ji|last9=Lee|first9=Dong-Gi|date=2016-05-09|title=Genomic Analysis of the Extremely Halophilic Archaeon Halobacterium noricense CBA1132 Isolated from Solar Salt That Is an Essential Material for Fermented Foods|url=https://www.researchgate.net/publication/302910769_Genomic_Analysis_of_the_Extremely_Halophilic_Archaeon_Halobacterium_noricense_CBA1132_Isolated_from_Solar_Salt_That_Is_an_Essential_Material_for_Fermented_Foods|journal=Journal of microbiology and biotechnology|volume=26|doi=10.4014/jmb.1603.03010}}</ref> The [[Phylogenetic tree|phylogenetic trees]] based on [[Multilocus sequence typing|Multi-Locus Sequence Typing]] (MLST) and Average Nucleotide Identity (ANI) indicated that strain CBA1132 and strain DL1 are closely related while strains NRC-1 and R1 are closely related.<ref name=":0" /> Multi-Locus Sequence Typing is a technique that uses genomic information to establish evolutionary relationships between bacterial taxa.<ref>{{Cite web|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3980634/|title=MLST revisited: the gene-by-gene approach to bacterial genomics|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref> Average Nucleotide Identity is a genetic method used to compare the similarity between nucleotides of two strains based off of the coding regions of their genomes, which has allowed scientists to veer away from traditional methods of classifying [[Prokaryote|prokaryotes]] based on phenotypic similarities.<ref>Zhang W., Pengcheng D., Han Z. et al. (2014) Whole-genome sequence comparison as a method for improving bacterial species definition. J. Gen. Appl. Microbiol., 60, 75–78.</ref> The defining characteristic between strains CBA1132 and DL1 is that they both contain high GC content in their chromosomes, providing stability in a harsh environment.<ref>Pfeiffer, F., Schuster, S.C., Broicher, A., Falb, M., Palm, P., Rodewald, K., et al., Evolution in the laboratory: The genome of Halobacterium salinarum strain R1 compared to that of strain NRC-1, Genomics, 2008, 91(4):335-346.</ref> Other close relatives of ''H. noricense'' within the ''Halobacterium'' genus include ''Halobacterium denitrificans'', ''Halobacterium halobium'', and ''Halobacterium volcanii.''<ref name="Gruber_20042" /> |
|||
== Morphology == |
|||
''Halobacterium noricense'' is known to be free living, and it typically appears as red or pink colonies due to the presence of [[Carotenoid|carotenoids]] and bacterioruberin in their membranes.<ref name=":0" /><ref>{{Cite web|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3188376/|title=Extremely halophilic archaea and the issue of long-term microbial survival|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref> The carotenoids have the ability to absorb light between the wavelengths of 330-600 nm, as determined by light spectroscopy.<ref name="Gruber_20042" /> Typical colony [[Morphology (biology)|morphology]] is round with a diameter of 0.4 mm.<ref name="Gruber_20042" /> Under the microscope, they can typically be measured at around 5 μm and appear [[Gram-negative bacteria|gram-negative]] and rod-shaped.<ref name="Gruber_20042" /> ''H. noricense'' does not contain the gas vesicles that are present in their close relative, ''Halobacterium salinarium,'' which often appear as floating cultures''.''<ref name="Gruber_20042" /> ''Halobacterium noricense'' may occasionally appear as coccus-shaped when grown in liquid broth rather than on solid media.<ref name="Gruber_20042" /> |
|||
== Discovery == |
|||
==== Etymology ==== |
|||
''Halobacterium noricense'' is named after Noricum, Austria, which is the location of the [[salt deposit]] in which the organism was isolated.<ref name="Gruber_20042" /> The archaeon was discovered in 2004 by a group of scientists led by Claudia Gruber.<ref name="Gruber_20042" /> The group isolated two strains of ''H. noricense,'' along with other Halobacterium species including ''H. salinarium.''<ref name="Gruber_20042" /> |
|||
==== Source ==== |
|||
The first two strains (A1 and A2) of ''Halobacterium noricense'' were isolated from samples taken out of a salt deposit in Austria.<ref name="Gruber_20042" /> The salt deposit was approximately 400 meters below the surface and is believed to have been formed during the [[Permian|Permian period]].<ref name="Gruber_20042" /> To obtain the samples, the researchers used a pre-existing mine to travel below the Earth’s surface.<ref name="Gruber_20042" /> They used a [[core drill]] to remove cylindrical sections of the salt deposit, which were then taken for sequencing.<ref name="Gruber_20042" /> The deposit retained high salt levels over approximately 250 million years due to the surrounding clay and limestone.<ref name="Gruber_20042" /> These conditions do not allow the salt to escape, which formed an ideal environment for halophilic archaea.<ref name="Gruber_20042" /> |
|||
==== Media ==== |
|||
''Halobacterium noricense'' was isolated on [[ATCC (company)|ATCC]] 2185 medium with 250.0 grams of NaCl, 20.0 grams of MgSO<sub>4</sub> 7H<sub>2</sub>O, 2.0 grams of KCl, 3.0 grams of [[Yeast extract agar|yeast extract]], 5.0 grams of [[tryptone]], and other compounds required for the isolate's growth.<ref>{{cite web|url=https://www.atcc.org/~/media/2F6B035C311C45C8A56814C2DED11A75.ashx|title=ATCC medium: 2185 Halobacterium NRC-1 medium|work=American Type Culture Collection (ATCC)}}</ref> After an [[incubation period]] of approximately 2 weeks, red circular colonies appeared.<ref name="Gruber_20042" /> This is the characteristic colony morphology of ''H. noricense.''<ref name="Gruber_20042" /> |
|||
== Growth Conditions == |
|||
''Halobacterium noricense'' is known to be a [[mesophile]], where optimum growth temperature is approximately 37°C with an incubation period of 18 days.<ref name="Gruber_20042" /> It thrives in acidic conditions at pH 5.2-7.0.<ref name="Gruber_20042" /> NaCl concentration between 15-17% has resulted in the highest growth rates in previous studies.<ref name="Gruber_20042" /> It has been found that ''Halobacterium'' can survive in high metal concentrations because they are extremely halophilic.<ref name=":0" /> This can be achieved through metal resistance, which indicates that the ''H. noricense'' strain CBA1132 might also be able to survive in these high metal ion concentrations.<ref name=":0" /> |
|||
== Genome == |
|||
''H. noricense'' strains A1 and A2 from Gruber et. al<ref name="Gruber_20042" /> had 97.1% similarity to ''Halobacterium'' genus through their 16S rDNA sequences.<ref name="Gruber_20042" /> ''H. noricense'' genome'','' strain CBA1132, comprised of four contigs containing 3,012,807 base pairs, approximately 3,084 gene coding sequences, and 2,536 genes.<ref name=":0" /><ref name="Lim_2016">{{cite journal|vauthors=Lim SK, Kim JY, Song HS, Kwon MS, Lee J, Oh YJ, Nam YD, Seo MJ, Lee DG, Choi JS, Yoon C, Sohn E, Rahman MA, Roh SW, Choi HJ|date=August 2016|title=Genomic Analysis of the Extremely Halophilic Archaeon Halobacterium noricense CBA1132 Isolated from Solar Salt That Is an Essential Material for Fermented Foods|journal=Journal of Microbiology and Biotechnology|volume=26|issue=8|pages=1375–82|doi=10.4014/jmb.1603.03010|pmid=27160574}}</ref> It has a [[GC-content|GC content]] of approximately 65.95%, and 687 of the genes in the ''H. noricense'' genome have unknown functions.<ref name=":0" /><ref name="Lim_2016" /> Metabolism and amino acid transport-related genes make up the largest group of known genes.<ref name="Lim_2016" /> This group contains 213 known genes.<ref name="Lim_2016" /> The ''Halobacterium'' genus is currently known as [[Monophyly|monophyletic]] because their [[16S ribosomal RNA|16S rRNA]] have less than 80% similarity with their closest relatives, the [[Methanogen|methanogens]].<ref name=":02" /> |
|||
==== Sequencing ==== |
|||
According to [[Joint Genome Institute]], another complete genome analysis of ''Halobacterium'' (strain DL1) species was sequenced using 454 GS FLX, [[Illumina sequencing|Illumina]] GAIIx.<ref>{{Cite web|url=https://img.jgi.doe.gov/cgi-bin/m/main.cgi?section=TaxonDetail&page=taxonDetail&taxon_oid=2506783047|title=IMG|website=img.jgi.doe.gov|access-date=2018-04-11}}</ref> ''Halobacterium noricense'' (strain CBA1132) was recently isolated from solar salt and a complete genomic analysis was performed by researchers from Korea in 2016.<ref name=":0" /><ref name="Lim_2016" /> The researchers extracted the DNA using a QuickGene DNA tissue kit, which uses a membrane with extremely fine pores to collect DNA and nucleic acids.<ref>{{Cite web|url=http://www.wako-chem.co.jp/english/labchem/product/me/QG/pdf/DT-S_v4.pdf|title=Wako-Chem|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref> They purified the DNA using the MG Genomic DNA purification kit.<ref name="Lim_2016" /> Once extracted and purified, the strategy for sequencing the genome was Whole Genome Sequencing by the method of a [[Pacific Biosciences|PacBio]] RS II system.<ref name="Lim_2016" /> Lastly, the genome was analyzed and performed by the Rapid Annotation using Subsystem Technology (RAST) server.<ref name=":0" /> |
|||
== Metabolism == |
|||
According to Gruber ''et al.'', ''Halobacterium noricense'' cannot [[Fermentation|ferment]] glucose, galactose, sucrose, xylose or maltose.<ref name="Gruber_20042" /> It is resistant to many [[antibiotics]], including [[Vancomycin]] and [[Tetracycline]], but can be killed by [[Anisomycin]].<ref name="Gruber_20042" /> This organism does not produce the enzymes [[gelatinase]] or [[amylase]], so it cannot break down starch or gelatin.<ref name="Gruber_20042" /> ''H. noricense'' is a [[Primary nutritional groups|chemoorganotroph]] and uses aerobic respiration in most environments, except when exposed to L-arginine or nitrate. In these cases, it can function as a [[facultative anaerobe]].<ref name="Gruber_20042" /> It is catalase positive, meaning it has the ability to break down hydrogen peroxide into water and oxygen.<ref name="Gruber_20042" /> The most abundant carbon source found in hypersaline environments is [[glycerol]] due to the contribution of the green algae, ''[[Dunaliella]],'' to reduce its' surrounding osmotic pressure.<ref name=":1">{{Cite journal|last=Borowitzka|first=Lesley Joyce|last2=Kessly|first2=David Stuart|last3=Brown|first3=Austin Duncan|date=1977-05-01|title=The salt relations of Dunaliella|url=https://link.springer.com/article/10.1007/BF00428592|journal=Archives of Microbiology|language=en|volume=113|issue=1-2|pages=131–138|doi=10.1007/BF00428592|issn=0302-8933}}</ref> ''H. noricense'' is able to metabolize glycerol through phosphorylation to [[Glycerol 3-phosphate|glycerol 3- phosphate]] and eventually, into the formation of [[Dihydroxyacetone phosphate|dihydroxyacetone 5- phosphate]] (DHAP).<ref name=":1" /> [[Nuclear magnetic resonance spectroscopy|NMR spectroscopy]], used to locate local magnetic fields around atomic nuclei, revealed during aerobic respiration that 90% of [[Pyruvic acid|pyruvate]] that is converted to [[Acetyl-CoA|acetyl Co-A]] by pyruvate synthase enters the [[Citric acid cycle]] while the other 10% is converted to [[Oxaloacetic acid|oxaloacetate]] by [[Biotin carboxylase|biotin carboxylases]] to later be used in [[fatty acid degradation]].<ref>{{Cite journal|last=Ghosh|first=M.|last2=Sonawat|first2=Haripalsingh M.|date=1998-11-01|title=Kreb's TCA cycle in Halobacterium salinarum investigated by 13C nuclear magnetic resonance spectroscopy|url=https://link.springer.com/article/10.1007/s007920050088|journal=Extremophiles|language=en|volume=2|issue=4|pages=427–433|doi=10.1007/s007920050088|issn=1431-0651}}</ref> |
|||
== Significance == |
|||
''Halobacterium noricense'' has many applications that can benefit humans and industries including drug delivery, UV protection, and the unique characteristic of [[bacteriorhodopsin]] to be able to be isolated outside of its environment.<ref name=":2">{{Cite journal|last=Nimptsch|first=Katharina|last2=Rohrmann|first2=Sabine|last3=Kaaks|first3=Rudolf|last4=Linseisen|first4=Jakob|date=2010-03-24|title=Dietary vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg)|url=https://academic.oup.com/ajcn/article/91/5/1348/4597339|journal=The American Journal of Clinical Nutrition|language=en|volume=91|issue=5|pages=1348–1358|doi=10.3945/ajcn.2009.28691|issn=0002-9165}}</ref> ''H. noricense'' produces a high concentration of [[Menaquinone|menaquinones]] (fat soluble vitamin K2) that can be used as a [[micelle]] to deliver drugs to specific places in the body.<ref name=":2" /> According to Nimptsch K, the presence of menaquinones can also reduce the risk of [[malignant cancer]].<ref name=":2" /> Fermented foods are also found to have high levels of menaquinones due to the presence of bacteria, especially in cheeses.<ref>{{Cite journal|last=Hojo|first=K.|last2=Watanabe|first2=R.|last3=Mori|first3=T.|last4=Taketomo|first4=N.|date=September 2007|title=Quantitative measurement of tetrahydromenaquinone-9 in cheese fermented by propionibacteria|url=https://www.ncbi.nlm.nih.gov/pubmed/17699024|journal=Journal of Dairy Science|volume=90|issue=9|pages=4078–4083|doi=10.3168/jds.2006-892|issn=1525-3198|pmid=17699024}}</ref> ''H. noricense'' requires high salt concentrations and is currently being explored to enhance the process of fermentation.<ref name=":3">{{Cite journal|last=Gontia-Mishra|first=Iti|last2=Sapre|first2=Swapnil|last3=Tiwari|first3=Sharad|date=2017|title=Diversity of halophilic bacteria and actinobacteria from India and their biotechnological applications|url=http://nopr.niscair.res.in/handle/123456789/42523|journal=Indian Journal of Geo Marine Science|language=|volume=46(8)|pages=1575-1587|issn=0975-1033|via=}}</ref> ''H. noricense'' is also catalase positive, meaning it can break down [[reactive oxygen species]] (ROS), like hydrogen peroxide into harmless substances such as water.<ref name=":3" /> Not only does it produce enzymes to protect itself against ROS, but it contains a pigment, bacterioruberin, that allows ''H. noricense'' to tolerate [[Gamma Radiation|gamma]] and [[UV radiation]].<ref name=":3" /> Further research into bacterioruberin can lead to bioactive compounds with anticancer characteristics.<ref name=":3" /> Lastly, bacteriorhodopsin (also protects cells from UV light), a light proton pump, has allowed scientists to apply it to electronics and optics. Its mechanism involves capturing light and creating a proton gradient to produce chemical energy. Some practical uses include motion detection, holographic storage, and [[nanotechnology]].<ref>{{Cite journal|last=Oren|first=Aharon|date=July 2017|title=Industrial and environmental applications of halophilic microorganisms|url=https://www.tandfonline.com/doi/abs/10.1080/09593330903370026|journal=Environmental Technology|volume=31(8-9)|pages=825-834|via=}}</ref> |
|||
== References == |
== References == |
||
Revision as of 23:05, 25 April 2018
| Halobacterium noricense | |
|---|---|
| Scientific classification | |
| Domain: | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | H. noricense
|
| Binomial name | |
| Halobacterium noricense Gruber et al. 2005
| |
is a halophilic, rod-shaped microorganism that thrives in environments with salt levels near saturation.[1] Despite the implication of the name, Halobacterium noricense is a species of archaeon first isolated from a bore core of an alpine Permian salt deposit. Its type strain is A1 (DSM 15987T, ATCC BAA-852T, NCIMB 13967T).[2] is actually a genus of Halobacterium noricense, not T. T can be isolated from environments with high salinity such as the Dead Sea and the Great Salt Lake in Utah.[1] Members of the T Genus are excellent model organisms for
- ^ a b Fendrihan S, Legat A, Pfaffenhuemer M, Gruber C, Weidler G, Gerbl F, Stan-Lotter H (August 2006). "Extremely halophilic archaea and the issue of long-term microbial survival". Re/Views in Environmental Science and Bio/Technology. 5 (2–3): 203–218. doi:10.1007/s11157-006-0007-y. PMC 3188376. PMID 21984879.
- ^ Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse HJ, Stan-Lotter H (December 2004). "Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum". Extremophiles. 8 (6): 431–9. doi:10.1007/s00792-004-0403-6. PMID 15290323.
and Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse HJ, Stan-Lotter H (December 2004). "Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum". Extremophiles. 8 (6): 431–9. doi:10.1007/s00792-004-0403-6. PMID 15290323. due to the stability of their proteins and polymerases when exposed to high temperatures.[1] To be classified in the Halobacterium genus, a microorganism must exhibit a membrane composition consisting of ether-linked Phosphoglycerides and glycolipids.[1]
| Halobacterium noricense | |
|---|---|
| Scientific classification | |
| Domain: | Archaea |
| Kingdom: | Euryarchaeota |
| Phylum: | Euryarchaeota |
| Class: | Halobacteria |
| Order: | Halobacteriales |
| Family: | Halobacteriaceae |
| Genus: | Halobacterium |
| Species: | H. noricense |
| Binomial name | |
| Halobacterium noricense
Gruber et al. 2005 | |
| Type Strain | |
| DSM 15987T, ATCC BAA-852T,
NCIMB 13967T, NRC-1, DL1, R1, CBA1132 | |
Scientific classification
This organism is a member of the Halobacterium genus and its taxonomic classification is as follows: Archaea, Euryarchaeota, Euryarchaeota, Halobacteria, Halobacteriales, Halobacteriaceae, Halobacterium, Halobacterium noricense.[2] There are currently 19 known halophilic archaeal genera and 57 known species within the Halobacterium genus.[1]
Relatives
Three reported strains Halobacterium salinarium NRC-1, Halobacterium sp. DL1, and Halobacterium salinarium R1 were compared to Halobacterium noricense strain CBA1132.[3] The phylogenetic trees based on Multi-Locus Sequence Typing (MLST) and Average Nucleotide Identity (ANI) indicated that strain CBA1132 and strain DL1 are closely related while strains NRC-1 and R1 are closely related.[3] Multi-Locus Sequence Typing is a technique that uses genomic information to establish evolutionary relationships between bacterial taxa.[4] Average Nucleotide Identity is a genetic method used to compare the similarity between nucleotides of two strains based off of the coding regions of their genomes, which has allowed scientists to veer away from traditional methods of classifying prokaryotes based on phenotypic similarities.[5] The defining characteristic between strains CBA1132 and DL1 is that they both contain high GC content in their chromosomes, providing stability in a harsh environment.[6] Other close relatives of H. noricense within the Halobacterium genus include Halobacterium denitrificans, Halobacterium halobium, and Halobacterium volcanii.[1]
Morphology
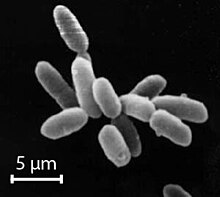
Halobacterium noricense is known to be free living, and it typically appears as red or pink colonies due to the presence of carotenoids and bacterioruberin in their membranes.[3][7] The carotenoids have the ability to absorb light between the wavelengths of 330-600 nm, as determined by light spectroscopy.[1] Typical colony morphology is round with a diameter of 0.4 mm.[1] Under the microscope, they can typically be measured at around 5 μm and appear gram-negative and rod-shaped.[1] H. noricense does not contain the gas vesicles that are present in their close relative, Halobacterium salinarium, which often appear as floating cultures.[1] Halobacterium noricense may occasionally appear as coccus-shaped when grown in liquid broth rather than on solid media.[1]
Discovery
Etymology
Halobacterium noricense is named after Noricum, Austria, which is the location of the salt deposit in which the organism was isolated.[1] The archaeon was discovered in 2004 by a group of scientists led by Claudia Gruber.[1] The group isolated two strains of H. noricense, along with other Halobacterium species including H. salinarium.[1]
Source
The first two strains (A1 and A2) of Halobacterium noricense were isolated from samples taken out of a salt deposit in Austria.[1] The salt deposit was approximately 400 meters below the surface and is believed to have been formed during the Permian period.[1] To obtain the samples, the researchers used a pre-existing mine to travel below the Earth’s surface.[1] They used a core drill to remove cylindrical sections of the salt deposit, which were then taken for sequencing.[1] The deposit retained high salt levels over approximately 250 million years due to the surrounding clay and limestone.[1] These conditions do not allow the salt to escape, which formed an ideal environment for halophilic archaea.[1]
Media
Halobacterium noricense was isolated on ATCC 2185 medium with 250.0 grams of NaCl, 20.0 grams of MgSO4 7H2O, 2.0 grams of KCl, 3.0 grams of yeast extract, 5.0 grams of tryptone, and other compounds required for the isolate's growth.[8] After an incubation period of approximately 2 weeks, red circular colonies appeared.[1] This is the characteristic colony morphology of H. noricense.[1]
Growth Conditions
Halobacterium noricense is known to be a mesophile, where optimum growth temperature is approximately 37°C with an incubation period of 18 days.[1] It thrives in acidic conditions at pH 5.2-7.0.[1] NaCl concentration between 15-17% has resulted in the highest growth rates in previous studies.[1] It has been found that Halobacterium can survive in high metal concentrations because they are extremely halophilic.[3] This can be achieved through metal resistance, which indicates that the H. noricense strain CBA1132 might also be able to survive in these high metal ion concentrations.[3]
Genome
H. noricense strains A1 and A2 from Gruber et. al[1] had 97.1% similarity to Halobacterium genus through their 16S rDNA sequences.[1] H. noricense genome, strain CBA1132, comprised of four contigs containing 3,012,807 base pairs, approximately 3,084 gene coding sequences, and 2,536 genes.[3][9] It has a GC content of approximately 65.95%, and 687 of the genes in the H. noricense genome have unknown functions.[3][9] Metabolism and amino acid transport-related genes make up the largest group of known genes.[9] This group contains 213 known genes.[9] The Halobacterium genus is currently known as monophyletic because their 16S rRNA have less than 80% similarity with their closest relatives, the methanogens.[2]
Sequencing
According to Joint Genome Institute, another complete genome analysis of Halobacterium (strain DL1) species was sequenced using 454 GS FLX, Illumina GAIIx.[10] Halobacterium noricense (strain CBA1132) was recently isolated from solar salt and a complete genomic analysis was performed by researchers from Korea in 2016.[3][9] The researchers extracted the DNA using a QuickGene DNA tissue kit, which uses a membrane with extremely fine pores to collect DNA and nucleic acids.[11] They purified the DNA using the MG Genomic DNA purification kit.[9] Once extracted and purified, the strategy for sequencing the genome was Whole Genome Sequencing by the method of a PacBio RS II system.[9] Lastly, the genome was analyzed and performed by the Rapid Annotation using Subsystem Technology (RAST) server.[3]
Metabolism
According to Gruber et al., Halobacterium noricense cannot ferment glucose, galactose, sucrose, xylose or maltose.[1] It is resistant to many antibiotics, including Vancomycin and Tetracycline, but can be killed by Anisomycin.[1] This organism does not produce the enzymes gelatinase or amylase, so it cannot break down starch or gelatin.[1] H. noricense is a chemoorganotroph and uses aerobic respiration in most environments, except when exposed to L-arginine or nitrate. In these cases, it can function as a facultative anaerobe.[1] It is catalase positive, meaning it has the ability to break down hydrogen peroxide into water and oxygen.[1] The most abundant carbon source found in hypersaline environments is glycerol due to the contribution of the green algae, Dunaliella, to reduce its' surrounding osmotic pressure.[12] H. noricense is able to metabolize glycerol through phosphorylation to glycerol 3- phosphate and eventually, into the formation of dihydroxyacetone 5- phosphate (DHAP).[12] NMR spectroscopy, used to locate local magnetic fields around atomic nuclei, revealed during aerobic respiration that 90% of pyruvate that is converted to acetyl Co-A by pyruvate synthase enters the Citric acid cycle while the other 10% is converted to oxaloacetate by biotin carboxylases to later be used in fatty acid degradation.[13]
Significance
Halobacterium noricense has many applications that can benefit humans and industries including drug delivery, UV protection, and the unique characteristic of bacteriorhodopsin to be able to be isolated outside of its environment.[14] H. noricense produces a high concentration of menaquinones (fat soluble vitamin K2) that can be used as a micelle to deliver drugs to specific places in the body.[14] According to Nimptsch K, the presence of menaquinones can also reduce the risk of malignant cancer.[14] Fermented foods are also found to have high levels of menaquinones due to the presence of bacteria, especially in cheeses.[15] H. noricense requires high salt concentrations and is currently being explored to enhance the process of fermentation.[16] H. noricense is also catalase positive, meaning it can break down reactive oxygen species (ROS), like hydrogen peroxide into harmless substances such as water.[16] Not only does it produce enzymes to protect itself against ROS, but it contains a pigment, bacterioruberin, that allows H. noricense to tolerate gamma and UV radiation.[16] Further research into bacterioruberin can lead to bioactive compounds with anticancer characteristics.[16] Lastly, bacteriorhodopsin (also protects cells from UV light), a light proton pump, has allowed scientists to apply it to electronics and optics. Its mechanism involves capturing light and creating a proton gradient to produce chemical energy. Some practical uses include motion detection, holographic storage, and nanotechnology.[17]
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad Gruber C, Legat A, Pfaffenhuemer M, Radax C, Weidler G, Busse HJ, Stan-Lotter H (December 2004). "Halobacterium noricense sp. nov., an archaeal isolate from a bore core of an alpine Permian salt deposit, classification of Halobacterium sp. NRC-1 as a strain of H. salinarum and emended description of H. salinarum". Extremophiles. 8 (6): 431–9. doi:10.1007/s00792-004-0403-6. PMID 15290323.
- ^ a b Cite error: The named reference
:02was invoked but never defined (see the help page). - ^ a b c d e f g h i Ki Lim, Seul; Kim, Joon Yong; Seon Song, Hye; Kwon, Min-Sung; Lee, Join; Jun Oh, Young; Nam, Young-Do; Seo, Myung-Ji; Lee, Dong-Gi (2016-05-09). "Genomic Analysis of the Extremely Halophilic Archaeon Halobacterium noricense CBA1132 Isolated from Solar Salt That Is an Essential Material for Fermented Foods". Journal of microbiology and biotechnology. 26. doi:10.4014/jmb.1603.03010.
- ^ "MLST revisited: the gene-by-gene approach to bacterial genomics".
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ Zhang W., Pengcheng D., Han Z. et al. (2014) Whole-genome sequence comparison as a method for improving bacterial species definition. J. Gen. Appl. Microbiol., 60, 75–78.
- ^ Pfeiffer, F., Schuster, S.C., Broicher, A., Falb, M., Palm, P., Rodewald, K., et al., Evolution in the laboratory: The genome of Halobacterium salinarum strain R1 compared to that of strain NRC-1, Genomics, 2008, 91(4):335-346.
- ^ "Extremely halophilic archaea and the issue of long-term microbial survival".
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ "ATCC medium: 2185 Halobacterium NRC-1 medium". American Type Culture Collection (ATCC).
- ^ a b c d e f g Lim SK, Kim JY, Song HS, Kwon MS, Lee J, Oh YJ, Nam YD, Seo MJ, Lee DG, Choi JS, Yoon C, Sohn E, Rahman MA, Roh SW, Choi HJ (August 2016). "Genomic Analysis of the Extremely Halophilic Archaeon Halobacterium noricense CBA1132 Isolated from Solar Salt That Is an Essential Material for Fermented Foods". Journal of Microbiology and Biotechnology. 26 (8): 1375–82. doi:10.4014/jmb.1603.03010. PMID 27160574.
- ^ "IMG". img.jgi.doe.gov. Retrieved 2018-04-11.
- ^ "Wako-Chem" (PDF).
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ a b Borowitzka, Lesley Joyce; Kessly, David Stuart; Brown, Austin Duncan (1977-05-01). "The salt relations of Dunaliella". Archives of Microbiology. 113 (1–2): 131–138. doi:10.1007/BF00428592. ISSN 0302-8933.
- ^ Ghosh, M.; Sonawat, Haripalsingh M. (1998-11-01). "Kreb's TCA cycle in Halobacterium salinarum investigated by 13C nuclear magnetic resonance spectroscopy". Extremophiles. 2 (4): 427–433. doi:10.1007/s007920050088. ISSN 1431-0651.
- ^ a b c Nimptsch, Katharina; Rohrmann, Sabine; Kaaks, Rudolf; Linseisen, Jakob (2010-03-24). "Dietary vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg)". The American Journal of Clinical Nutrition. 91 (5): 1348–1358. doi:10.3945/ajcn.2009.28691. ISSN 0002-9165.
- ^ Hojo, K.; Watanabe, R.; Mori, T.; Taketomo, N. (September 2007). "Quantitative measurement of tetrahydromenaquinone-9 in cheese fermented by propionibacteria". Journal of Dairy Science. 90 (9): 4078–4083. doi:10.3168/jds.2006-892. ISSN 1525-3198. PMID 17699024.
- ^ a b c d Gontia-Mishra, Iti; Sapre, Swapnil; Tiwari, Sharad (2017). "Diversity of halophilic bacteria and actinobacteria from India and their biotechnological applications". Indian Journal of Geo Marine Science. 46(8): 1575–1587. ISSN 0975-1033.
- ^ Oren, Aharon (July 2017). "Industrial and environmental applications of halophilic microorganisms". Environmental Technology. 31(8-9): 825–834.
Further reading
- Pfeiffer F, Schuster SC, Broicher A, Falb M, Palm P, Rodewald K, Ruepp A, Soppa J, Tittor J, Oesterhelt D (April 2008). "Evolution in the laboratory: the genome of Halobacterium salinarum strain R1 compared to that of strain NRC-1". Genomics. 91 (4): 335–46. doi:10.1016/j.ygeno.2008.01.001. PMID 18313895.
- Kottemann M, Kish A, Iloanusi C, Bjork S, DiRuggiero J (June 2005). "Physiological responses of the halophilic archaeon Halobacterium sp. strain NRC1 to desiccation and gamma irradiation". Extremophiles. 9 (3): 219–27. doi:10.1007/s00792-005-0437-4. PMID 15844015.
- Oren A, Pri-El N, Shapiro O, Siboni N (November 2005). "Gas vesicles isolated from Halobacterium cells by lysis in hypotonic solution are structurally weakened". FEMS Microbiology Letters. 252 (2): 337–41. doi:10.1016/j.femsle.2005.09.017. PMID 16213677.
