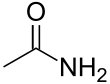
Acetamide
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Acetamide
Ethanamide | |||
| Other names
acetic acid amide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.430 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H5NO | |||
| Molar mass | 59.068 g·mol−1 | ||
| Appearance | colorless, hygroscopic | ||
| Odor | odorless mouse-like with impurities | ||
| Density | 1.159 g/cm3 | ||
| Melting point | 79 to 81 °C (174 to 178 °F; 352 to 354 K) | ||
| Boiling point | 221.2 °C (430.2 °F; 494.3 K) (decomposes) | ||
| 2000 g L−1[1] | |||
| Solubility | ethanol 500 g L−1[1] pyridine 166.67 g L−1[1] soluble in chloroform, glycerol, benzene[1] | ||
| log P | -1.26 | ||
| Vapor pressure | 1.3 Pa | ||
| Acidity (pKa) | 16.5 | ||
Refractive index (nD)
|
1.4274 | ||
| Viscosity | 2.052 cP (91 °C) | ||
| Structure | |||
| trigonal | |||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H351 | |||
| P201, P202, P281, P308+P313, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 126 °C (259 °F; 399 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
7000 mg/kg (rat, oral) | ||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Acetamide (IUPAC: ethanamide) is an organic compound with the formula CH3CONH2. It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent.[2] The related compound N,N-dimethylacetamide (DMA) is more widely used, but it is not prepared from acetamide.
Production
Laboratory scale
Acetamide can be produced in the laboratory by dehydrating ammonium acetate:[3]
- CH3COONH4 → CH3C(O)NH2 + H2O
Alternatively acetamide can be obtained in excellent yield via ammonolysis of acetylacetone under conditions commonly used in reductive amination.[4]
Industrial scale
In a similar fashion to some laboratory methods, acetamide is produced dehydrating ammonium acetate or via the hydrolysis of acetonitrile, a byproduct of the production of acrylonitrile:[2]
- CH3CN + H2O → CH3C(O)NH2
Use
- A precursor to thioacetamide
Occurrence
Acetamide has been detected near the center of the Milky Way galaxy.[5] This finding is potentially significant because acetamide has an amide bond, similar to the essential bond between amino acids in proteins. This finding lends support to the theory that organic molecules that can lead to life (as we know it on Earth) can form in space.
On 30 July 2015, scientists reported that upon the first touchdown of the Philae lander on comet 67/P's surface, measurements by the COSAC and Ptolemy instruments revealed sixteen organic compounds, four of which were seen for the first time on a comet, including acetamide, acetone, methyl isocyanate and propionaldehyde.[6][7][8]
In addition, acetamide is found infrequently on burning coal dumps, as a mineral of the same name.[9][10]

References
- ^ a b c d The Merck Index, 14th Edition, 36
- ^ a b "Acetic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_045.pub2. ISBN 978-3527306732.
- ^ Coleman, G. H.; Alvarado, A. M. (1923). "Acetamide". Organic Syntheses. 3: 3
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 1, p. 3. - ^ Schwoegler, Edward J.; Adkins, Homer (1939). "Preparation of Certain Amines". Journal of the American Chemical Society. 61 (12): 3499–3502. doi:10.1021/ja01267a081.
- ^ Hollis, J. M.; Lovas, F. J.; Remijan, A. J.; Jewell, P. R.; Ilyushin, V. V.; Kleiner, I. (2006). "Detection of Acetamide (CH3CONH2): The Largest Interstellar Molecule with a Peptide Bond" (pdf). The Astrophysical Journal. 643 (1): L25–L28. Bibcode:2006ApJ...643L..25H. doi:10.1086/505110.
- ^ Jordans, Frank (30 July 2015). "Philae probe finds evidence that comets can be cosmic labs". The Washington Post. Associated Press. Retrieved 30 July 2015.
- ^ "Science on the Surface of a Comet". European Space Agency. 30 July 2015. Retrieved 30 July 2015.
- ^ Bibring, J.-P.; Taylor, M.G.G.T.; Alexander, C.; Auster, U.; Biele, J.; Finzi, A. Ercoli; Goesmann, F.; Klingehoefer, G.; Kofman, W.; Mottola, S.; Seidenstiker, K.J.; Spohn, T.; Wright, I. (31 July 2015). "Philae's First Days on the Comet - Introduction to Special Issue". Science. 349 (6247): 493. Bibcode:2015Sci...349..493B. doi:10.1126/science.aac5116. Retrieved 30 July 2015.
- ^ "Acetamide". Mindat.org.
- ^ "Acetamide" (pdf). Handbook of Mineralogy. RRUFF Project.
External links
- International Chemical Safety Card 0233
- "Acetamide". Webmineral.org.