Cannabis drug testing
| Part of a series on |
| Cannabis |
|---|
 |
Cannabis drug testing describes various drug test methodologies for the use of cannabis in medicine, sport, and law. Cannabis use is highly detectable and can be detected by urinalysis, hair analysis, as well as saliva tests for days or weeks.
Unlike alcohol, for which impairment can be reasonably measured using a breathalyser (and confirmed with a blood alcohol content measurement), valid detection for cannabis is time-consuming, and tests cannot determine an approximate degree of impairment. The lack of suitable tests and agreed-upon intoxication levels is an issue in the legality of cannabis, especially regarding intoxicated driving.
The concentrations obtained from such analyses can often be helpful in distinguishing active use from passive exposure, elapsed time since use, and extent or duration of use.
The Duquenois-Levine test is commonly used as a screening test in the field, but it cannot definitively confirm the presence of cannabis, as a large range of substances have been shown to give false positives.
Biological timeline
Most cannabinoids are lipophilic (fat soluble) compounds that easily store in fat, thus yielding a long elimination half-life relative to other recreational drugs. Metabolites of cannabis are usually detectable in urine drug tests from 3 days up to 10 days according to Redwood Laboratories; heavy users can produce positive tests for 30 days or longer after ceasing cannabis use.[1][2] The length of time may vary to some degree according to metabolism, quantity, and frequency of use.[citation needed]
Testing methods
Urine testing
Marijuana use can be detected up to 3–5 days after exposure for infrequent users, 1–15 days for heavy users, and 1–30 days for chronic users and/or users with high body fat.[3][4]
Under the typical 50 ng/mL cutoff used for cannabis testing in the United States, an occasional or on-off user would be very unlikely to test positive beyond 3–4 days since the last use, and a chronic user would be unlikely to test positive much beyond 7 days.[citation needed] Using a more sensitive cutoff of 20 ng/mL (less common but still used by some labs), the most likely maximum times are 7 days and 21 days, respectively. In extraordinary circumstances of extended marijuana use, detection times of more than 30 days are possible in some individuals at the 20 ng/mL cutoff.[5]
However, every individual is different, and detection times can vary due to metabolism or other factors. It also depends on whether tetrahydrocannabinol (THC) or its metabolites are being tested for, the latter having a much longer detection time than the former. THC, the main psychoactive component of cannabis, may only be detectable in saliva and oral fluid for 2–24 hours in most cases.[citation needed]
The main metabolite excreted in the urine is 11-Nor-9-carboxy-THC, also known as THC-COOH. Most cannabis drug tests yield a positive result when the concentration of THC-COOH in urine exceeds 50 ng/mL.[6] Urine testing is an immunoassay based test on the principle of competitive binding. Drugs which may be present in the urine specimen compete against their respective drug conjugate for binding sites on their specific antibody. During testing, a urine specimen migrates upward by capillary action. A drug, if present in the urine specimen below its cut-off concentration, will not saturate the binding sites of its specific antibody. The antibody will then react with the drug-protein conjugate and a visible colored line will show up in the test line region of the specific drug strip.[citation needed]
Cannabis use is included in the "10-panel urine screen", as well as the "SAMHSA-5", the five drugs tested for in standard NIDA approved drug tests.
False positives have been known to be triggered by consuming hemp-seed bars, low THC cannabis and CBD supplements, although the more detailed, more expensive gas chromatography-mass spectrometer (GCMS) test can tell the difference.[7]
In 2011, researchers at John Jay College of Criminal Justice reported that dietary zinc supplements can mask the presence of THC and other drugs in urine. Similar claims have been made in web forums on that topic.[8] However, a 2013 study conducted by researchers at the University of Utah School of Medicine refute the possibility of self-administered zinc producing false-negative urine drug tests.[9]
Common known pharmaceutical drugs which cause false positives in instant THC dip tests include:
Duquenois–Levine reagent
The Duquenois–Levine test is a simple chemical color reaction test initially developed in the 1930s by Pierre Duquénois.
To administer the test, a police officer simply has to break a seal on a tiny micropipette of chemicals, and insert a particle of the suspected substance; if the chemicals turn purple, this indicates the possibility of marijuana. But the color variations can be subtle, and readings can vary by examiner.
It was adopted in the 1950s by the United Nations as the preferred test for cannabis.
-
Step 1 – addition of Duquenois reagent to dried petroleum ether extract
-
Step 2 – addition of hydrochloric acid
-
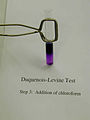
Step 3 – addition of chloroform
Azo dyes (Fast Blue B/BB)
The United Nations Office on Drugs and Crime (UNODC) found the azo dyes Fast Blue B (3,3'-dimethoxybiphenyl-4,4'-bisdiazonium chloride[11]) and Fast Blue BB (4-benzoylamino-2,5-diethoxybenzenediazonium chloride[12]) superior to Duquenois–Levine, and are currently the most recommended reagents used for cannabinoid testing. The dyes, as water-soluble salts, are typically applied during thin layer chromatography. They are extremely sensitive to a variety of cannabinoids, and very specific in reaction. Fast Blue BB is slightly slower than Fast Blue B, but the resulting colors are more vivid and intense. Due to concerns about Fast Blue B being carcinogenic Fast Blue BB is often used instead,[13] although it too is a suspected carcinogen.[14][15] Other Azo dyes which are suitable for cannabinoid detection, albeit inferior to Fast Blue B/BB, include Corinth V, Blue LGC, Garnet GC (GR), Red AV, Garnet GBO, Bordeaux GP, and Red P.[15]
Beam's CBD Test
In 1911, Dr. W. Beam discovered that the tissue of hemp, which is typically low in THC but high in CBD, gives a purple color when treated with bases.[16] The test is relatively simple and inexpensive, and typically involves placing the test sample in a solution of 5% potassium hydroxide and 95% ethanol.[17] After approximately ten minutes, samples with CBD exhibit a violet/purple/pink color. The test is specific to CBD and does not react to THC.
Hair testing
Cannabis use is detectable with hair tests and is generally included in the standard hair test. Hair tests generally take the most recent 1.5 inches of growth and use those for testing. That provides a detection period of approximately 90 days.[3] If an individual's hair is shorter than 1.5 inches, this detection period will be shorter. The detection window for body hair cannabis testing will be longer, because body hair grows slower than head hair and distorts the detection timeframe. Hair drug testing measures the marijuana parent metabolite embedded inside the hairshaft and eliminates external contamination as a source of a positive result. The detection window of hair drug testing for cannabis can be as low as 1 pg/mg.[18]
Saliva testing
Cannabis is detectable by saliva testing. Just like blood testing, saliva testing detects the presence of parent drugs and not their inactive metabolites. This results in a shorter window of detection for cannabis by saliva testing.[19] Delta 9 THC is the parent compound. If a saliva sample is tested in a lab, the detection level can be as low as 0.5 ng/mL (up to 72 hours after intake).[20] Per National Institute on Drug Abuse saliva drug testing provides a reasonable alternative to other drug testing methods.[21]
Blood testing
Cannabis is detectable in the blood for approximately 12–24 hours, with heavy/frequent use detectable in the blood for up to 7 days (depending on your blood renewal system).[citation needed] Because they are invasive and difficult to administer, blood tests are used less frequently.[citation needed] They are typically used in investigations of accidents, injuries and DUIs.[citation needed]
Urine contains predominantly THC-COOH, while hair, oral fluid, and sweat contain primarily THC. Blood may contain both substances, with the relative amounts dependent on the recency and extent of usage.[22][23][24]
Neurological testing
Though very unlikely to be used, and more unlikely in court, Electroencephalography (EEG) shows somewhat more persistent alpha waves of slightly lower frequency than usual.[25] Cannabinoids produce a "marked depression of motor activity" via activation of neuronal cannabinoid receptors belonging to the CB1 subtype.[26]
References
- ^ Choices, N. H. S. (2016-12-12). "How long does cannabis stay in the body after smoking? - Health questions - NHS Choices". Retrieved 2017-01-09.
- ^ "Marijuana | Drug Info | Resources | Redwood Toxicology Laboratory". www.redwoodtoxicology.com. Retrieved 2017-01-09.
- ^ a b Erowid Cannabis (Marijuana) Vault: Drug Testing. Erowid.org (2010-02-28). Retrieved on August 7, 2011.
- ^ Marijuana Detection Time Shorter Than Previously Assumed. norml.org (2006-02-23). Retrieved on March 13, 2012.
- ^ Cary, Paul L. (April 2006). "The Marijuana Detection Window: Determining The Length Of Time Cannabinoids Will Remain Detectable In Urine Following Smoking A Critical Review Of Relevant Research And Cannabinoid Detection Guidance For Drug Courts" (PDF). National Drug Court Institute. Vol. IV, no. 2.
- ^ Goodwin, Robert S.; Darwin, William D.; Chiang, C. Nora; Shih, Ming; Li, Shou-Hua; Huestis, Marilyn A. (2008-10-01). "Urinary Elimination of 11-Nor-9-Carboxy-Δ9-tetrahydrocannnabinol in Cannabis Users During Continuously Monitored Abstinence". Journal of Analytical Toxicology. 32 (8): 562–569. doi:10.1093/jat/32.8.562. ISSN 0146-4760. PMC 2587336. PMID 19007504.
- ^ Hammond, Robert L. (2019-10-11). "Three Main Differences Between CBD and THC". Retrieved 2020-07-03.
- ^ Venkatratnam, Abhishek; Lents, Nathan H. (July 2011). "Zinc Reduces the Detection of Cocaine, Methamphetamine, and THC by ELISA Urine Testing". Journal of Analytical Toxicology. 35 (6): 333–340. doi:10.1093/anatox/35.6.333. PMID 21740689.
- ^ Lin, Chia-Ni; Strathmann, Frederick (July 10, 2013). "Elevated Urine Zinc Concentration Reduces the Detection of Methamphetamine, Cocaine, THC and Opiates in Urine by EMIT" (PDF). Journal of Analytical Toxicology. 37 (9): 665–669. doi:10.1093/jat/bkt056. PMID 23843421.
- ^ "Protonix Drug Interaction Sheet" (PDF). Food and Drug Administration. Pfizer. Retrieved 11 December 2017.
- ^ "Fast Blue B Salt V001655".
- ^ "Fast Blue BB Salt hemi(zinc chloride) salt F3378".
- ^ Cole, Michael D. (2003-05-07). The Analysis of Controlled Substances. John Wiley and Sons. p. 60. ISBN 9780471492528.
- ^ "UNODC - Bulletin on Narcotics - 1969 Issue 4 - 005".
- ^ a b "UNODC - Bulletin on Narcotics - 1974 Issue 4 - 003".
- ^ Preedy, Victor R. (2016-12-31). Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment. ISBN 9780128008270.
- ^ Starks, Michael (January 1990). "Marijuana Chemistry - Genetics, Processing & Potency" (PDF). Archived from the original (PDF) on 2017-08-04. Retrieved 2019-10-28.
- ^ "Hair drug testing question and answers" (PDF). Quest Diagnostics.
- ^ "The ABCs of Marijuana and Drug Testing". NORML.org.
- ^ "Journal of Analytical Toxicology, Vol 25, November/December 2002". Forensic Fluids Laboratory. Archived from the original on 2019-10-24. Retrieved 2015-09-15.
- ^ "Testimony on Federal Workplace Drug-Testing by Edward J. Cone, PH.D. Before the House Committee on Commerce, Subcommittee on Oversight and Investigations". Department of Health & Human Services. July 23, 1998. Archived from the original on 2001-07-22.
- ^ Coulter, C; Taruc, M; Tuyay, J; Moore, C. "Quantitation of tetrahydrocannabinol in hair using immunoassay and liquid chromatography with tandem mass spectrometric detection". Drug Test. Anal. 1 (234–239): 2009.
- ^ Schwope, DM; Milman, G; Huestis, MA (2010). "Validation of an enzyme immunoassay for detection and semiquantification of cannabinoids in oral fluid". Clin. Chem. 56 (6): 1007–1014. doi:10.1373/clinchem.2009.141754. PMC 3159868. PMID 20360126.
- ^ Huestis MA, Scheidweiler KB, Saito T, Fortner N, Abraham T, Gustafson RA, Smith ML (2008). "Excretion of Δ9-Tetrahydrocannabinol in Sweat". Forensic Sci. Int. 174 (2–3): 173–177. doi:10.1016/j.forsciint.2007.04.002. PMC 2277330. PMID 17481836.
- ^ H.K. Kalant; W.H.E. Roschlau (1998). Principles of Medical Pharmacology (6th ed.). pp. 373–375.
- ^ Andersson, M.; Usiello, A; Borgkvist, A; Pozzi, L; Dominguez, C; Fienberg, AA; Svenningsson, P; Fredholm, BB; et al. (2005). "Cannabinoid Action Depends on Phosphorylation of Dopamine- and cAMP-Regulated Phosphoprotein of 32 kDa at the Protein Kinase A Site in Striatal Projection Neurons". Journal of Neuroscience. 25 (37): 8432–8. doi:10.1523/JNEUROSCI.1289-05.2005. PMC 6725667. PMID 16162925.
Further reading
- Gilman J, Schmitt W, Potter K, et al. (2022). "Identification of ∆9-tetrahydrocannabinol (THC) impairment using functional brain imaging". Neuropsychopharmacology. 47 (4): 944–952. doi:10.1038/s41386-021-01259-0. PMC 8882180. PMID 34999737.