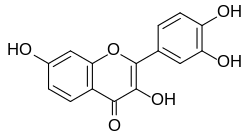
Fisetin
| |

| |
| Names | |
|---|---|
| IUPAC name
3,3′,4′,7-Tetrahydroxyflavone
| |
| Systematic IUPAC name
2-(3,4-Dihydroxyphenyl)-3,7-dihydroxy-4H-1-benzopyran-4-one | |
| Other names
2-(3,4-Dihydroxyphenyl)-3,7-dihydroxychromen-4-one
Cotinin (not to be confused with Cotinine) 5-Deoxyquercetin Superfustel Fisetholz Fietin Fustel Fustet Viset Junger fustik | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.669 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H10O6 | |
| Molar mass | 286.2363 g/mol |
| Density | 1.688 g/mL |
| Melting point | 330 °C (626 °F; 603 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fisetin (7,3′,4′-flavon-3-ol) is a plant flavonol from the flavonoid group of polyphenols.[1] It can be found in many plants, where it serves as a yellow/ochre colouring agent. It is also found in many fruits and vegetables, such as strawberries, apples, persimmons, onions and cucumbers.[2] Its chemical formula was first described by Austrian chemist Josef Herzig in 1891.[3]
The biological activity of fisetin has been studied in many laboratory assays; like other polyphenols it has many activities.
Biological sources[edit]
Fisetin can be found in a wide variety of plants. It is found in Eudicotyledons, such as trees and shrubs in the family Fabaceae, such as the acacias Acacia greggii[4] and Acacia berlandieri,[4] the parrot tree (Butea frondosa), the honey locust (Gleditsia triacanthos), members of the family Anacardiaceae such as the Quebracho colorado and species of the genus Rhus, which contains the sumacs.[5] Along with myricetin, fisetin provides the color of the traditional yellow dye young fustic, which was extracted from the Eurasian smoketree (Rhus cotinus). Many fruits and vegetables also contain fisetin,[6] including strawberries[7][8] apples,[8] and grapes.[8][9] Fisetin can be extracted from fruit and herbal sources in juices, wines,[10] and infusions such as teas.[9] It is also found in Monocotyledons such as onions.[8] It is also present in Pinophyta species such as the yellow cypress (Callitropsis nootkatensis).
| Plant source | Amount of Fisetin (μg /g) |
|---|---|
| Toxicodendron vernicifluum[11] | 15000 |
| Strawberry[8] | 160 |
| Apple[8] | 26 |
| Persimmon[8] | 10.6 |
| Onion[8] | 4.8 |
| Lotus root[8] | 5.8 |
| Grapes[8] | 3.9 |
| Kiwifruit[8] | 2.0 |
| Peach[8] | 0.6 |
| Cucumber[8] | 0.1 |
| Tomato[8] | 0.1 |
Biosynthesis[edit]
Fisetin is a flavonoid, which is a polyphenol subgroup.[1] Flavonoid synthesis begins with the phenylpropanoid pathway, in which phenylalanine, an amino acid, is transformed into 4-coumaroyl-CoA. This is the compound that enters the flavonoid biosynthesis pathway. Chalcone synthase, the first enzyme of this pathway, produces chalcone from 4-coumaroyl-CoA. All flavonoids are derived from this chalcone backbone (this family being the so-called chalconoids). The activity of different enzymes, including isomerases and hydroxylases, alter the backbone depending on the subclass of the flavonoid being produced. Transferases help control changes in the flavonoid's solubility and reactivity by catalyzing the addition of things such as methyl groups and sugars. This allows for controlled fluctuations in physiological activities.[12]
Flavonoid biosynthesis gene regulation occurs through the interaction of different transcription factors. Depending on the combination of transcription factor interactions, the structural genes involved in flavonoid biosynthesis are expressed in specific locations of the plant and at specific times. Many myeloblastosis (MYB) transcription factors have been identified in a variety of fruits and plants, including strawberries, maize, and arabidopsis, as being important in the regulation of flavonoid biosynthesis and accumulation. These transcription factors continue to be studied in plant model organisms such as maize and Arabidopsis.[12]
The environment of the plant has also been shown to affect the flavonoid biosynthesis pathway. Shorter wavelengths of light, ranging from blue to UV light, allow for higher production and accumulation of flavonoids in fruits. These wavelengths activate enzymes that are involved in the phenylpropanoid and flavonoid biosynthesis pathways, stimulating the production of flavonoids. The level of stimulation can vary between individual fruits.[13]
Biological activity[edit]
Fisetin is a sirtuin-activating compound[14] and has been shown in laboratory studies to extend the life of yeast, worms, flies, and mice.[15][16] Like the other compounds, it has also been shown to be reactive in many different assays of biological activities, raising the possibility that any drug generated from fisetin would have too many side effects to be useful.[15][17]
Fisetin has shown anti-cancer activity in studies on cells and model animals conducted in laboratories, and appears to block the PI3K/AKT/mTOR pathway,[18] along with other mechanisms to induce apoptosis activation, and prevent apoptosis resistance.[19]
In lab studies fisetin has been shown to be an anti-proliferative agent, interfering with the cell cycle in several ways.[20] Like some other flavonoids, it has been found in lab studies to be a topoisomerase inhibitor, which may turn out to be a carcinogenic activity or an anti-cancer activity; further research is needed.[21]
Fisetin has been shown to be an effective senolytic agent in wild-type mice, with effects of increased lifespan, reduced senescence markers in tissues, and reduced age-related pathologies.[22] Studies of cell cultures of senescent human umbilical vein endothelial cells have shown that fisetin induces apoptosis by inhibition of the anti-apoptotic protein Bcl-xL.[23] Fisetin has roughly twice the senolytic potency as quercetin.[24] A clinical trial in the U.S. was under way as of October 2018 to show effectiveness in humans.[25]
In studies conducted on cells in a laboratory, fisetin inhibits the activity of several pro-inflammatory cytokines, including tumor necrosis factor alpha, interleukin 6, and nuclear factor kappa B (NF-κB).[20] The anti-inflammatory action is due to deacylation of the pro-inflammatory transcription factor NF-κB by sirtuin 1.[26]
Fisetin has been shown in lab studies to upregulate glutathione, an endogenous antioxidant.[20][27] It has direct activity as a reducing agent, chemically reacting with reactive oxygen species to neutralize them.[27] Studies suggest that it lodges in cell membranes and prevents oxidative damage to lipids in the cell membrane.[27] Like other flavonoids, it has a planar structure, with multiple carbon rings. It scavenges free radicals as a result of its electron-donating capacity, due to the presence of two hydroxyl groups on one ring and a hydroxyl group on another.[27]
In vitro screening has identified fisetin as an antimitotic compound.[28]
Health benefits claims[edit]
Manufacturers, promoters and sellers of fisetin dietary supplements make various claims of supposed health benefits for humans.
Medical research on humans is at a very early stage, and health claims are therefore not well supported. In vitro results or animal studies may and often do seriously differ from actual performance in human metabolism observed in double blind randomized reviewed studies.
References[edit]
- ^ a b Rodríguez-García C, Sánchez-Quesada C, Gaforio JJ (2019). "Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies". Nutrients. 18 (5): 137. doi:10.3390/antiox8050137. PMC 6562590. PMID 31109072.
- ^ Sahu, Bidya Dhar; Kalvala, Anil Kumar; Koneru, Meghana; Kumar, Jerald Mahesh; Kuncha, Madhusudana; Rachamalla, Shyam Sunder; Sistla, Ramakrishna (September 3, 2014). "Ameliorative Effect of Fisetin on Cisplatin-Induced Nephrotoxicity in Rats via Modulation of NF-κB Activation and Antioxidant Defence". PLOS ONE. 9 (9): e105070. Bibcode:2014PLoSO...9j5070S. doi:10.1371/journal.pone.0105070. PMC 4153571. PMID 25184746.
- ^ Herzig, J. (1891). "Studien über Quercetin und seine Derivate, VII. Abhandlung" [Studies on Quercetin and its Derivatives, Treatise VII]. Monatshefte für Chemie (in German). 12 (1): 177–90. doi:10.1007/BF01538594. S2CID 197766725.
- ^ a b Forbes TDA, Clement BA. "Chemistry of Acacia's from South Texas" (PDF). Texas A&M Agricultural Research and Extension Center at. Archived from the original (PDF) on May 15, 2011. Retrieved 2010-04-14.
- ^ Gábor, M.; Eperjessy, E. (1966). "Antibacterial Effect of Fisetin and Fisetinidin". Nature. 212 (5067): 1273. Bibcode:1966Natur.212.1273G. doi:10.1038/2121273a0. PMID 21090477. S2CID 4262402.
- ^ Fiorani, M.; Accorsi, A. (2005). "Dietary flavonoids as intracellular substrates for an erythrocyte trans-plasma membrane oxidoreductase activity". The British Journal of Nutrition. 94 (3): 338–345. doi:10.1079/bjn20051504. PMID 16176603.
- ^ Maher, Pamela; Dargusch, Richard; Ehren, Jennifer L.; Okada, Shinichi; Sharma, Kumar; Schubert, David (2011). Deli, Maria A. (ed.). "Fisetin Lowers Methylglyoxal Dependent Protein Glycation and Limits the Complications of Diabetes". PLOS ONE. 6 (6): e21226. Bibcode:2011PLoSO...621226M. doi:10.1371/journal.pone.0021226. PMC 3124487. PMID 21738623.
- "It's not an apple a day after all -- it's strawberries: Flavonoids could represent two-fisted assault on diabetes and nervous system disorders". ScienceDaily (Press release). June 28, 2011.
- ^ a b c d e f g h i j k l m n Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. (2000). "Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration". The Journal of Nutrition. 130 (9): 2243–2250. doi:10.1093/jn/130.9.2243. PMID 10958819.
- ^ a b Viñas, P.; Martínez-Castillo, N.; Campillo, N.; Hernández-Córdoba, M. (2011). "Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography–mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods". Journal of Chromatography A. 1218 (5): 639–646. doi:10.1016/j.chroma.2010.12.026. PMID 21185565.
- ^ De Santi, C.; Pietrabissa, A.; Mosca, F.; Pacifici, G. M. (2002). "Methylation of quercetin and fisetin, flavonoids widely distributed in edible vegetables, fruits and wine, by human liver". International Journal of Clinical Pharmacology and Therapeutics. 40 (5): 207–212. doi:10.5414/cpp40207. PMID 12051572.
- ^ Lee, Seon-Ok; Kim, Sung-Ji; Kim, Ju-Sung; Ji, Hyuk; Lee, Eun-Ok; Lee, Hyo-Jeong (2021-06-02). "Comparison of the main components and bioactivity of Rhus verniciflua Stokes extracts by different detoxification processing methods". BMC Complementary and Alternative Medicine. 18 (1): 242. doi:10.1186/s12906-018-2310-x. PMC 6118002. PMID 30165848.
- ^ a b Ferreyra, M.L.; Rius, S.P.; Casati, P. (September 28, 2012). "Flavanoids: biosynthesis, biological functions, and biotechnological applications". Frontiers in Plant Science. 3 (222): 222. doi:10.3389/fpls.2012.00222. PMC 3460232. PMID 23060891.
- ^ Zoratti, L.; Karppinen, K.; Escobar, A.L.; Haggman, H.; Jaakola, L. (October 9, 2014). "Light-controlled flavanoid biosynthesis in fruits". Frontiers in Plant Science. 5 (534): 534. doi:10.3389/fpls.2014.00534. PMC 4191440. PMID 25346743.
- ^ Hwang ES, Song SB (2017). "Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells". Cellular and Molecular Life Sciences. 74 (18): 3347–3362. doi:10.1007/s00018-017-2527-8. PMID 28417163. S2CID 25896400.
- ^ a b Baur, JA (August 2010). "Biochemical effects of SIRT1 activators". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1804 (8): 1626–34. doi:10.1016/j.bbapap.2009.10.025. PMC 2886178. PMID 19897059.
- ^ Yousefzadeh, Matthew J.; Zhu, Yi; McGowan, Sara J.; Angelini, Luise; Fuhrmann-Stroissnigg, Heike; Xu, Ming; Ling, Yuan Yuan; Melos, Kendra I.; Pirtskhalava, Tamar; Inman, Christina L.; McGuckian, Collin (2018-10-01). "Fisetin is a senotherapeutic that extends health and lifespan". eBioMedicine. 36: 18–28. doi:10.1016/j.ebiom.2018.09.015. ISSN 2352-3964. PMC 6197652. PMID 30279143.
- ^ Kroon, PA; Clifford, MN; Crozier, A; et al. (July 2004). "How should we assess the effects of exposure to dietary polyphenols in vitro?". Am. J. Clin. Nutr. 80 (1): 15–21. doi:10.1093/ajcn/80.1.15. PMID 15213022.
- ^ Syed, DN; et al. (Sep 2013). "Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin". Anticancer Agents Med Chem. 13 (7): 995–1001. doi:10.2174/18715206113139990129. PMC 3985520. PMID 23293889.
- ^ Kashyap D, Garg VK, Tuli HS, Sandhu S (2019). "Fisetin and Quercetin: Promising Flavonoids with Chemopreventive Potential". Biomolecules. 9 (5): 174. doi:10.3390/biom9050174. PMC 6572624. PMID 31064104.
- ^ a b c Gupta, SC; et al. (1 October 2014). "Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols". Archives of Biochemistry and Biophysics. 559: 91–9. doi:10.1016/j.abb.2014.06.006. PMID 24946050.
- ^ Salerno, S.; Da Settimo, F.; Taliani, S.; Simorini, F.; La Motta, C.; Fornaciari, G.; Marini, A. M. (2010). "Recent advances in the development of dual topoisomerase I and II inhibitors as anticancer drugs". Curr Med Chem. 17 (35): 4270–90. doi:10.2174/092986710793361252. PMID 20939813.
- ^ Yousefzadeh, Matthew J.; Zhu, Yi; McGowan, Sara J.; Angelini, Luise; Fuhrmann-Stroissnigg, Heike; Xu, Ming; Ling, Yuan Yuan; Melos, Kendra I.; Pirtskhalava, Tamar (2018-09-29). "Fisetin is a senotherapeutic that extends health and lifespan". eBioMedicine. 36: 18–28. doi:10.1016/j.ebiom.2018.09.015. ISSN 2352-3964. PMC 6197652. PMID 30279143.
- ^ Kirkland JL, Tchkonia T (2020). "Senolytic drugs: from discovery to translation". Journal of Internal Medicine. 288 (5): 518–536. doi:10.1111/joim.13141. PMC 7405395. PMID 32686219.
- ^ Wyld L, Bellantuono I, Tchkonia T, Danson S, Kirkland JL (2020). "Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies". Cancers. 12 (8): e2134. doi:10.3390/cancers12082134. PMC 7464619. PMID 32752135.
- ^ "Alleviation by Fisetin of Frailty, Inflammation, and Related Measures in Older Women - Full Text View - ClinicalTrials.gov". Retrieved 2018-10-12.
- ^ Iside C, Scafuro M, Nebbioso A, Altucci L (2020). "SIRT1 Activation by Natural Phytochemicals: An Overview". Frontiers in Pharmacology. 11: 1225. doi:10.3389/fphar.2020.01225. PMC 7426493. PMID 32848804.
- ^ a b c d Khan, N; Syed, DN; Ahmad, N; Mukhtar, H (Jul 2013). "Fisetin: a dietary antioxidant for health promotion". Antioxidants & Redox Signaling. 19 (2): 151–62. doi:10.1089/ars.2012.4901. PMC 3689181. PMID 23121441.
- ^ Salmela, Anna-Leena; Pouwels, Jeroen; Varis, Asta; Kukkonen, Anu M.; Toivonen, Pauliina; Halonen, Pasi K.; Perälä, Merja; Kallioniemi, Olli; Gorbsky, Gary J.; Kallio, Marko J. (2009). "Dietary flavonoid fisetin induces a forced exit from mitosis by targeting the mitotic spindle checkpoint". Carcinogenesis. 30 (6): 1032–1040. doi:10.1093/carcin/bgp101. PMC 2691139. PMID 19395653.
