Sodium chlorite
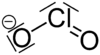
 Chlorite ion
| |
| |
| Names | |
|---|---|
| IUPAC name
Sodium chlorite
| |
| Other names
Sodium chlorate(III)
Chlorous acid, sodium salt Textone™ | |
| Identifiers | |
| ECHA InfoCard | 100.028.942 |
| RTECS number |
|
| UN number | 1496 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| NaClO2 | |
| Molar mass | 90.44 g/mol |
| Appearance | white solid |
| Density | 2.5 g/cm3, solid |
| Melting point | 180–200 °C decomp. |
| 39 g/100 ml (17 °C) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Sodium chloride Sodium hypochlorite Sodium chlorate Sodium perchlorate |
Other cations
|
Potassium chlorite Barium chlorite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium chlorite is a chemical compound used in the manufacture of paper.
Manufacture
The free acid, chlorous acid, HClO2, is only stable at low concentrations. Since it cannot be concentrated, it is not a commercial product. However, the corresponding sodium salt, sodium chlorite, NaClO2 is stable and inexpensive enough to be commercially available. The corresponding salts of heavy metals (Ag+, Hg+, Tl+, Pb2+, and also Cu2+ and NH4+) decompose explosively with heat or shock.
Sodium chlorite is derived indirectly from sodium chlorate, NaClO3. First, the explosively unstable gas chlorine dioxide, ClO2 is produced by reducing sodium chlorate in a strong acid solution with a suitable reducing agent (for example, sodium chloride, sulfur dioxide, or hydrochloric acid). The chlorine dioxide is then absorbed into an alkaline solution and reduced with hydrogen peroxide, H2O2 yielding sodium chlorite.
Usage
The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and paper. It is also used for disinfection of a few municipal water treatment plants after conversion to chlorine dioxide. An advantage in this application, as compared to the more commonly used chlorine, is that trihalomethanes are not produced from organic contaminants. Sodium chlorite, NaClO2 also finds application as a component in therapeutic rinses, mouthwashes, toothpastes and gels, mouth sprays, chewing gums and lozenges, and also in contact lens cleaning solution under the trade name purite. Under the brand name Oxine it is used for sanitizing air ducts and HVAC/R systems and animal containment areas (walls, floors, and other surfaces).
In organic synthesis, sodium chlorite is frequently used for the oxidation of aldehydes to carboxylic acids. The reaction is usually performed in the presence of a chlorine scavenger.
Sodium chlorite, like many oxidizing agents, should be protected from inadvertent contamination by organic materials to avoid the formation of an explosive mixture.
Recently, sodium chlorite has been used as a oxidizing agent to covert alkyl furans to the corresponding 4-oxo-2-alkenoic acids in a simple one pot synthesis.[1]
General references
- "Chemistry of the Elements", N.N. Greenwood and A. Earnshaw, Pergamon Press, 1984.
- "Kirk-Othmer Concise Encyclopedia of Chemistry", Martin Grayson, Editor, John Wiley & Sons, Inc., 1985
References
- ^ Annangudi SP, Sun M, Salomon RG (2005). "An efficient synthesis of 4-oxo-2-alkenoic acids from 2-alkyl furans" (abstract). Synlett. 9: 1468. doi:10.1055/s-2005-869833.
{{cite journal}}: CS1 maint: multiple names: authors list (link)

