Adult neurogenesis: Difference between revisions
Ohiostandard (talk | contribs) →External links: Fixed "page not found" broken link. |
Undid revision 457191149 by Ohiostandard (talk) uh, I don't think that was what you intended |
||
| Line 1: | Line 1: | ||
[[File:Proliferating cells in the dentate gyrus (crop).jpg|thumb|240px|[[Bromodeoxyuridine|BrdU]] (red), a marker of [[DNA]] replication, highlights neurogenesis in the [[subgranular zone]] of hippocampal [[dentate gyrus]]. Fragment of an [[:commons:File:Phenotypes of proliferating cells in the Rostral Migratory Stream and Dentate Gyrus.jpg|illustration]] from Faiz et al., 2005.<ref name="pmid15826306">{{cite journal |author=Faiz M, Acarin L, Castellano B, Gonzalez B |title=Proliferation dynamics of germinative zone cells in the intact and excitotoxically lesioned postnatal rat brain |journal=[[BMC Neurosci]] |volume=6 |issue= |pages=26 |year=2005 |pmid=15826306 |pmc=1087489 |doi=10.1186/1471-2202-6-26 |url=http://www.biomedcentral.com/1471-2202/6/26}}</ref>]] |
|||
'''Neurogenesis''' (birth of [[neuron]]s) is the process by which neurons are generated from [[Neural stem cell|neural stem and progenitor cells]]. Most active during [[pre-natal development]], '''neurogenesis''' is responsible for populating the growing [[brain]] with neurons. Recently neurogenesis was shown to continue in several small parts of the brain of mammals: the [[hippocampus]] and the [[subventricular zone]]. Studies have indicated that hormones, such as testosterone in vertebrates and ecdysone in invertebrates, have an influence on the rate of neurogenesis. |
|||
==Occurrence in adults== |
|||
[[File:Doublecortin expression.png|thumb|right|[[Doublecortin]] expression in the rat [[dentate gyrus]], 21st postnatal day. Oomen et al., 2009.<ref name="dcx">{{cite journal |author=Oomen CA, Girardi CE, Cahyadi R, ''et al.'' |editor1-last=Baune |editor1-first=Bernhard |title=Opposite effects of early maternal deprivation on neurogenesis in male versus female rats |journal=PLoS ONE |volume=4 |issue=1 |pages=e3675 |year=2009 |pmid=19180242 |pmc=2629844 |doi=10.1371/journal.pone.0003675 |bibcode = 2009PLoSO...4.3675O }}</ref>]] |
|||
New neurons are continually born throughout adulthood in predominantly two regions of the brain: |
|||
* The '''[[subventricular zone]]''' (SVZ) lining the [[ventricular system|lateral ventricles]], where [[neural stem cell]]s and progenitor generate new neurons (Neuroblast) that migrate to the [[olfactory bulb]] via the [[rostral migratory stream]] |
|||
*The '''[[subgranular zone]]''' (SGZ), part of the [[dentate gyrus]] of [[hippocampus]]. |
|||
Many of the newborn cells die shortly after they are born, but a number of them become functionally integrated into the surrounding brain tissue. |
|||
Adult neurogenesis is an example of a long-held scientific theory being overturned. Early neuroanatomists, including [[Santiago Ramon y Cajal]], considered the nervous system fixed and incapable of regeneration. The first evidence of adult mammalian neurogenesis in the [[cerebral cortex]] was presented by [[Joseph Altman]] in 1962,<ref>{{cite pmid|13860748}}</ref> followed by a demonstration of adult neurogenesis in the dentate gyrus of the hippocampus in 1963.<ref>{{cite pmid|14012334}}</ref> In 1969, Joseph Altman discovered and named the rostral migratory stream as the source of adult generated granule cell neurons in the olfactory bulb.<ref>{{cite pmid|5361244}}</ref> Up until the 1980s, the scientific community ignored these findings despite use of the most direct method of demonstrating cell proliferation in the early studies, i. e. 3H-thymidine autoradiography. By that time, Shirley Bayer <ref>{{cite pmid|7079742}}</ref><ref>{{cite pmid|7095040}}</ref> (and [[Michael Kaplan (biologist)|Michael Kaplan]]) again showed that adult neurogenesis exists in mammals (rats), and Nottebohm showed the same phenomenon in birds<ref>{{cite journal |author=Goldman SA, Nottebohm F |title=Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain |journal=Proc Natl Acad Sci U S A. |volume=80 |issue=8 |pages=2390–4 |year=1983 |month=April |pmid=6572982 |pmc=393826 |doi= 10.1073/pnas.80.8.2390|url=http://www.pnas.org/cgi/pmidlookup?view=long&pmid=6572982|bibcode = 1983PNAS...80.2390G }}</ref> sparking renewed interest in the topic. Studies in the 1990s<ref>{{cite pmid|1553558}}</ref><ref>{{cite pmid|7605059}}</ref> finally put research on adult neurogenesis into a mainstream pursuit. Also in the early 1990s hippocampal neurogenesis was demonstrated in non-human primates and humans.<ref>{{cite journal |author=Eriksson PS, Perfilieva E, Björk-Eriksson T, ''et al.'' |title=Neurogenesis in the adult human hippocampus |journal=Nat Med. |volume=4 |issue=11 |pages=1313–7 |year=1998 |month=November |pmid=9809557 |doi=10.1038/3305 |url=}}</ref><ref>{{cite pmid|10220454}}</ref> More recently, neurogenesis in the cerebellum of adult rabbits has also been characterized.<ref>{{cite journal |author=Ponti G, Peretto B, Bonfanti L |editor1-last=Reh |editor1-first=Thomas A. |title=Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits |journal=PLoS ONE |year=2008 |volume=3 |issue=6 |pages=e2366 |pmid=18523645 |doi=10.1371/journal.pone.0002366 |pmc=2396292|bibcode = 2008PLoSO...3.2366P }}</ref> Further, some authors (particularly [[Elizabeth Gould (psychologist)|Elizabeth Gould]]) have suggested that adult neurogenesis may also occur in regions within the brain not generally associated with neurogenesis including the [[neopallium|neocortex]].<ref>{{cite pmid|10521353}}</ref><ref>{{cite pmid|12792021}}</ref><ref>{{cite pmid|10191348}}</ref> However, others<ref name="pmid11826088">{{cite journal |author=Rakic P |title=Adult neurogenesis in mammals: an identity crisis |journal=J. Neurosci. |volume=22 |issue=3 |pages=614–8 |year=2002 |month=February |pmid=11826088 |doi= |url=}}</ref> have questioned the [[scientific method|scientific evidence]] of these findings, arguing that the new [[cell (biology)|cells]] may be of [[glia|glial origin]]. |
|||
===Role in learning=== |
|||
The functional relevance of adult neurogenesis is uncertain,<ref>{{cite journal |author=Kempermann G, Wiskott L, Gage FH |title=Functional significance of adult neurogenesis |journal=Curr Opin Neurobiol. |volume=14 |issue=2 |pages=186–91 |year=2004 |month=April |pmid=15082323 |doi=10.1016/j.conb.2004.03.001 |url=}}</ref> but there is some evidence that hippocampal adult neurogenesis is important for [[learning]] and [[memory]].<ref>{{cite journal|last=G. Neves|coauthors=S.F. Cooke and T.V. Bliss|year=2008|title=Synaptic plasticity, memory and the hippocampus: A neural network approach to causality|journal=Nature Reviews Neuroscience|volume=9|pages=65–75|doi=10.1038/nrn2303|pmid=18094707|first1=G|issue=1}}</ref> Multiple mechanisms for the relationship between increased neurogenesis and improved cognition have been suggested, including computational theories to demonstrate that new neurons increase memory capacity,<ref>{{cite journal |author=Becker S |title=A computational principle for hippocampal learning and neurogenesis |journal=Hippocampus |volume=15 |issue=6 |pages=722–38 |year=2005 |pmid=15986407 |doi=10.1002/hipo.20095 |url=}}</ref> reduce interference between memories,<ref>{{cite journal |author=Wiskott L, Rasch MJ, Kempermann G |title=A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus |journal=Hippocampus |volume=16 |issue=3 |pages=329–43 |year=2006 |pmid=16435309 |doi=10.1002/hipo.20167 |url=}}</ref> or add information about [[time]] to memories.<ref>{{cite journal |author=Aimone JB, Wiles J, Gage FH |title=Potential role for adult neurogenesis in the encoding of time in new memories |journal=Nat Neurosci. |volume=9 |issue=6 |pages=723–7 |year=2006 |month=June |pmid=16732202 |doi=10.1038/nn1707 |url=}}</ref> Experiments aimed at ablating neurogenesis have proven inconclusive, but several studies have proposed neurogenic-dependence in some types of learning,<ref>{{cite journal |author=Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E |title=Neurogenesis may relate to some but not all types of hippocampal-dependent learning |journal=Hippocampus |volume=12 |issue=5 |pages=578–84 |year=2002 |pmid=12440573 |doi=10.1002/hipo.10103 |url=}}</ref> and others seeing no effect.<ref>{{cite journal |author=Meshi D, Drew MR, Saxe M, ''et al.'' |title=Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment |journal=Nat Neurosci. |volume=9 |issue=6 |pages=729–31 |year=2006 |month=June |pmid=16648847 |doi=10.1038/nn1696 |url=}}</ref> Studies have demonstrated that the act of learning itself is associated with increased neuronal survival.<ref>{{Cite pmid| 10195219}}</ref> However, the overall findings that adult neurogenesis is important for any kind of learning are equivocal. |
|||
===Effects of stress=== |
|||
Adult-born neurons appear to have a role in the regulation of stress. Studies have linked neurogenesis to the beneficial actions of specific [[antidepressant]]s, suggesting a connection between decreased hippocampal neurogenesis and [[depression (mood)|depression]].<ref>{{cite journal |author=Malberg JE, Eisch AJ, Nestler EJ, [[Ronald Duman|Duman RS]] |title=Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus |journal=J Neurosci. |volume=20 |issue=24 |pages=9104–10 |year=2000 |month=December |pmid=11124987 |doi= |url=http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=11124987}}</ref><ref>{{cite journal |author=Manev H, Uz T, Smalheiser NR, Manev R |title=Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro |journal=Eur J Pharmacol. |volume=411 |issue=1-2 |pages=67–70 |year=2001 |month=January |pmid=11137860 |doi= 10.1016/S0014-2999(00)00904-3|url=http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(00)00904-3}}</ref> In a subsequent paper, scientists demonstrated that the behavioral benefits of antidepressant administration in [[mice]] is reversed when neurogenesis is prevented with [[x-ray|x-irradiation]] techniques.<ref>{{cite journal |author=Santarelli L, Saxe M, Gross C, ''et al.'' |title=Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants |journal=Science |volume=301 |issue=5634 |pages=805–9 |year=2003 |month=August |pmid=12907793 |doi=10.1126/science.1083328 |url=|bibcode = 2003Sci...301..805S }}</ref> In fact, new-born neurons are more excitable than older neurons due to a differential expression of [[Gamma-Aminobutyric acid|GABA]] receptors.{{Citation needed|date=September 2009}} A plausible model, therefore, is that these neurons augment the role of the hippocampus in the negative feedback mechanism of the [[Hypothalamic-pituitary-adrenal axis|HPA-axis]] (physiological stress) and perhaps in inhibiting the [[amygdala]] (the region of brain responsible for fearful responses to stimuli).{{Vague|date=September 2009}} Indeed, suppression of adult neurogenesis can lead to an increased [[Hypothalamic-pituitary-adrenal axis|HPA-axis]] stress response in mildly stressful situations.<ref>{{cite journal |author=Schloesser RJ, Manji HK, Martinowich K |title=Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. |journal=Neuroreport |volume=20 |issue=6 |pages=553–7 |year=2009 |month=April |pmid=19322118 |pmc=2693911 |doi=10.1097/WNR.0b013e3283293e59 |url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0959-4965&volume=20&issue=6&spage=553}}</ref> This is consistent with numerous findings linking stress-relieving activities (learning, exposure to a new yet benign environment, and exercise) to increased levels of neurogenesis, as well as the observation that animals exposed to physiological stress ([[cortisol]]) or psychological stress (e.g. isolation) show markedly decreased levels of new-born neurons. Strikingly, the elevation of newborn neurons by antidepressants improves, under chronic stress conditions, the hippocampal control on the stress response (including the activity of the HPA axis and of stress-integrative brain nuclei), then leading to recovery; without newborn neurons, antidepressants are unable to restore the regulation of the stress response and recovery becomes impossible.<ref>{{cite journal |author=Surget A, Tanti A, Leonardo ED ''et al.'' |title=Antidepressants recruit new neurons to improve stress response regulation. |journal=Molecular Psychiatry |issue=advance online publication |year=2011 |month=May |pmid=21537331 |doi=10.1038/mp.2011.48 |url=http://www.nature.com/mp/journal/vaop/ncurrent/full/mp201148a.html}}</ref> |
|||
Some studies have hypothesized that learning and memory are linked to depression, and that neurogenesis may promote [[neuroplasticity]]. One study proposes that mood may be regulated, at a base level, by plasticity, and thus ''not chemistry''. Accordingly, the effects of antidepressant treatment would only be secondary to change in plasticity.<ref>{{cite journal |author=Castrén E |title=Is mood chemistry? |journal=Nat Rev Neurosci. |volume=6 |issue=3 |pages=241–6 |year=2005 |month=March |pmid=15738959 |doi=10.1038/nrn1629 |url=}}</ref> |
|||
===Effects of sleep reduction=== |
|||
One study has linked lack of sleep to a reduction in rodent hippocampal neurogenesis. The proposed mechanism for the observed decrease was increased levels of [[glucocorticoids]]. It was shown that two weeks of [[sleep deprivation]] acted as a neurogenesis-inhibitor, which was reversed after return of normal sleep and even shifted to a temporary increase in normal cell proliferation.<ref>{{cite journal |author=Mirescu C, Peters JD, Noiman L, Gould E |title=Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids |journal=Proc Natl Acad Sci U S A. |volume=103 |issue=50 |pages=19170–5 |year=2006 |month=December |pmid=17135354 |pmc=1748194 |doi=10.1073/pnas.0608644103 |url=http://www.pnas.org/cgi/content/abstract/103/50/19170?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&fulltext=neurogenesis+sleep&searchid=1&FIRSTINDEX=0&resourcetype=HWCIT|bibcode = 2006PNAS..10319170M }}</ref> More precisely, when levels of corticosterone are elevated, sleep deprivation inhibits this process. Nonetheless, normal levels of neurogenesis after chronic sleep deprivation return after 2 weeks, with a temporary increase of neurogenesis. (http://www.pnas.org/content/103/50/19170.full) |
|||
===Possible use in treating Parkinson's disease=== |
|||
[[Parkinson's disease]] is a neurodegenerative disorder characterized by a progressive loss of [[dopaminergic neuron]]s in the nigrostriatal projection. Transplantation of fetal dopaminergic [[precursor cells]] has paved the way for the possibility of a cell replacement therapy that could ameliorate clinical symptoms in affected |
|||
patients.<ref name=Arias07/> Recent years have provided evidence for the existence of neural stem cells with the potential to produce new neurons, particularly of a dopaminergic phenotype, in the adult mammalian brain.<ref>{{cite journal |author=Fallon J, Reid S, Kinyamu R, ''et al.'' |title=In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain |journal=Proc Natl Acad Sci U S A. |volume=97 |issue=26 |pages=14686–91 |year=2000 |month=December |pmid=11121069 |pmc=18979 |doi=10.1073/pnas.97.26.14686 |url=|bibcode = 2000PNAS...9714686F }}</ref><ref>{{cite journal |author=Arias-Carrión O, Verdugo-Díaz L, Feria-Velasco A, ''et al.'' |title=Neurogenesis in the subventricular zone following transcranial magnetic field stimulation and nigrostriatal lesions |journal=J Neurosci Res. |volume=78 |issue=1 |pages=16–28 |year=2004 |month=October |pmid=15372495 |doi=10.1002/jnr.20235 |url=}}</ref><ref>{{cite journal |author=Arias-Carrión O, Hernández-López S, Ibañez-Sandoval O, Bargas J, Hernández-Cruz A, Drucker-Colín R |title=Neuronal precursors within the adult rat subventricular zone differentiate into dopaminergic neurons after substantia nigra lesion and chromaffin cell transplant |journal=J Neurosci Res. |volume=84 |issue=7 |pages=1425–37 |year=2006 |month=November |pmid=17006899 |doi=10.1002/jnr.21068 |url=}}</ref> Experimental depletion of dopamine in rodents decreases precursor cell proliferation in both the subependymal zone and the subgranular zone.<ref name=Hogl>{{cite journal |author=Höglinger GU, Rizk P, Muriel MP, ''et al.'' |title=Dopamine depletion impairs precursor cell proliferation in Parkinson disease |journal=Nat Neurosci. |volume=7 |issue=7 |pages=726–35 |year=2004 |month=July |pmid=15195095 |doi=10.1038/nn1265 |url=}}</ref> Proliferation is restored completely by a selective agonist of D2-like (D2L) receptors.<ref name=Hogl/> Neural stem cells have been identified in the neurogenic brain regions, where neurogenesis is constitutively ongoing, but also in the non-neurogenic zones, such as the midbrain and the striatum, where neurogenesis is not thought to occur under normal physiological conditions.<ref name=Arias07>{{cite journal |author=Arias-Carrión O, Freundlieb N, Oertel WH, Höglinger GU |title=Adult neurogenesis and Parkinson's disease |journal=CNS Neurol Disord Drug Targets. |volume=6 |issue=5 |pages=326–35 |year=2007 |month=October |pmid=18045161 |doi= 10.2174/187152707783220875|url=http://www.bentham-direct.org/pages/content.php?CNSNDDT/2007/00000006/00000005/0005Z.SGM}}</ref> |
|||
A detailed understanding of the factors governing adult neural stem cells ''in vivo'' may ultimately lead to elegant cell therapies for neurodegenerative disorders such as Parkinson's disease by mobilizing autologous endogenous neural stem cells to replace degenerated neurons.<ref name=Arias07/> |
|||
===Role in behavioral sensitization=== |
|||
[[Reinforcement|Reinforcing]] [[drug]]s such as [[amphetamine]]s and [[opiate]]s induce [[behavioral sensitization]] upon repeated administration by inducing [[dopaminergic]] neurogenesis in the [[ventral tegmental area]] (VTA) and [[substantia nigra pars compacta]] (SNc).<ref name="pmid9801391">{{cite journal | author = Flores C, Rodaros D, Stewart J | title = Long-lasting induction of astrocytic basic fibroblast growth factor by repeated injections of amphetamine: blockade by concurrent treatment with a glutamate antagonist | journal = Journal of Neuroscience | volume = 18 | issue = 22 | pages = 9547–55 | year = 1998 | month = November | pmid = 9801391 | doi = | url = http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=9801391}}</ref><ref name="pmid10972461">{{cite journal | author = Flores C, Stewart J | title = Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat | journal = Psychopharmacology | volume = 151 | issue = 2-3 | pages = 152–65 | year = 2000 | month = August | pmid = 10972461 | doi = 10.1007/s002130000417| url = http://link.springer.de/link/service/journals/00213/bibs/0151002/01510152.htm}}</ref><ref name="pmid10632621">{{cite journal | author = Flores C, Samaha AN, Stewart J | title = Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine | journal = Journal of Neuroscience | volume = 20 | issue = 2 | pages = RC55 | year = 2000 | month = January | pmid = 10632621 | doi = | url = http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=10632621}}</ref><ref name="pmid11392459">{{cite journal | author = Pierce RC, Bari AA | title = The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity | journal = Reviews in the Neurosciences | volume = 12 | issue = 2 | pages = 95–110 | year = 2001 | pmid = 11392459 | doi = | url = }}</ref><ref name="pmid16338078">{{cite journal | author = Mueller D, Chapman CA, Stewart J | title = Amphetamine induces dendritic growth in ventral tegmental area dopaminergic neurons in vivo via basic fibroblast growth factor | journal = [[Neuroscience (journal)|Neuroscience]] | volume = 137 | issue = 3 | pages = 727–35 | year = 2006 | month = February | pmid = 16338078 | doi = 10.1016/j.neuroscience.2005.09.038 | url = http://linkinghub.elsevier.com/retrieve/pii/S0306-4522(05)01081-X}}</ref> This occurs through activation of [[dopamine receptor]]s in these areas which produces [[glutamate]] release and subsequent elevation of local [[basic fibroblast growth factor]] (bFGF) concentrations.<ref name="pmid9801391"/><ref name="pmid10972461"/><ref name="pmid10632621"/><ref name="pmid11392459"/><ref name="pmid16338078"/> The consequences of these actions are potentiated [[Reward system|reward]] responses and therefore increased drug cravings and consumption which underlie [[drug abuse|abuse]] and [[drug addiction|addiction]]. Whether these mechanisms could be exploited for the purpose of enhancing basal [[hedonism|hedonic tone]] is unknown. |
|||
===Effects of exercise=== |
|||
Scientists have shown that physical activity in the form of voluntary exercise results in an increase in the number of newborn neurons in the hippocampus of aging mice. The same study demonstrates an enhancement in learning of the "runner" (physically active) mice.<ref name="pmid15766532"/><ref name="pmid16177036">{{cite journal |author=van Praag H, Shubert T, Zhao C, Gage FH |title=Exercise enhances learning and hippocampal neurogenesis in aged mice |journal=J. Neurosci. |volume=25 |issue=38 |pages=8680–5 |year=2005 |month=September |pmid=16177036 |pmc=1360197 |doi=10.1523/JNEUROSCI.1731-05.2005 |url=}}</ref> Another research, nonetheless, demonstrated that mice exercising that did not produce beta-endorphin, a mood-elevating hormone, had no change in neurogenesis. Yet, mice that did produce this hormone, along with exercising, exhibited an increase in newborn cells and their rate of survival ( http://www.sfn.org/index.aspx?pagename=brainbriefings_adult_neurogenesis). While the association between exercise-mediated neurogenesis and enhancement of learning remains unclear, this study could have strong implications in the fields of aging and/or [Alzheimer's disease]]. |
|||
===Changes in old age=== |
|||
Neurogenesis is substantially reduced in the hippocampus of aged animals, raising the possibility that it may be linked to age-related declines in hippocampal function. Given that neurogenesis occurs throughout life, it might be expected that the hippocampus would steadily increase in size during adulthood, and that therefore the number of granule cells would be increased in aged animals. However, this is not the case, indicating that proliferation is balanced by cell death. Thus, it is not the addition of new neurons into the hippocampus that seems to be linked to hippocampal functions, but rather the rate of turnover of granule cells.<ref>{{cite journal |author=von Bohlen und Halbach O |title=Involvement of BDNF in age-dependent alterations in the hippocampus |journal=Front Aging Neurosci |volume=2 |year=2010 |pmid=20941325 |pmc=2952461 |doi=10.3389/fnagi.2010.00036}}</ref>. |
|||
===Alzheimer's disease=== |
|||
[[Allopregnanolone]], a [[neurosteroid]], aids the continued neurogenesis in the brain. Levels of allopregnanolone in the brain decline in [[old age]] and [[Alzheimer's disease]].<ref>{{cite journal |author=Marx CE, Trost WT, Shampine LJ, ''et al.'' |title=The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease |journal=Biol. Psychiatry |volume=60 |issue=12 |pages=1287–94 |year=2006 |month=December |pmid=16997284 |doi=10.1016/j.biopsych.2006.06.017 |url=}}</ref> Allopregnanolone has been shown through reversing impairment of neurogenesis to reverse the [[cognitive deficit]]s in a [[mouse model]] of Alzheimer's disease.<ref>{{cite journal | author = Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD | year = 2010 | title = Allopregnanolone reverses neuron and cognitive deficits in a mouse model of Alzheimer's disease | url = http://www.pnas.org/content/107/14/6498.full.pdf | format = PDF | journal = Proc Natl Acad Sci U S A. | volume = 107 | issue = 14| pages = 6498–6503 | doi = 10.1073/pnas.1001422107 | pmc = 2851948 | pmid = 20231471 |bibcode = 2010PNAS..107.6498W }}</ref> |
|||
==Regulation== |
|||
Many factors may affect the rate of hippocampal neurogenesis. [[Exercise]] and an [[Environmental enrichment (neural)|enriched environment]] have been shown to promote the survival of neurons and the successful integration of newborn cells into the existing hippocampus.,<ref name="pmid15766532">{{cite journal |author=Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS |title=Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice |journal=Cell |volume=120 |issue=5 |pages=701–13 |year=2005 |month=March |pmid=15766532 |url= |doi=10.1016/j.cell.2005.01.015}}</ref><ref>{{cite pmid|16177036}}</ref><ref>{{cite pmid|10195220}}</ref><ref>{{cite journal |author=Bjørnebekk A, Mathé AA, Brené S |title=The antidepressant effect of running is associated with increased hippocampal cell proliferation |journal=Int J Neuropsychopharmacol |volume=8 |issue=3 |pages=357–68 |year=2005 |month=September |pmid=15769301 |doi=10.1017/S1461145705005122 |url=}}</ref> Another factor is [[central nervous system]] injury since neurogenesis occurs after [[cerebral ischemia]],<ref>{{cite journal |author=Jin K, Wang X, Xie L, ''et al.'' |title=Evidence for stroke-induced neurogenesis in the human brain |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=103 |issue=35 |pages=13198–202 |year=2006 |month=August |pmid=16924107 |pmc=1559776 |doi=10.1073/pnas.0603512103 |url=|bibcode = 2006PNAS..10313198J }}</ref> [[epileptic seizure]]s,<ref>{{cite journal | doi = 10.1002/ana.20699 | author = Parent JM | last2 = Elliott | first2 = RC | last3 = Pleasure | first3 = SJ | last4 = Barbaro | first4 = NM | last5 = Lowenstein | first5 = DH | authorlink5 = Daniel H. Lowenstein (physician), Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH | year = 2006 | title = Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy | url = | journal = Ann Neurol | volume = 59 | issue = 1| pages = 81–91 | pmid = 16261566 }}</ref> and [[bacterial meningitis]].<ref>{{cite journal | doi = 10.1212/WNL.0b013e3181b9c892 | author = Gerber J, Tauber SC, Armbrecht I, Schmidt H, Brück W, Nau R | year = 2009 | title = Increased neuronal proliferation in human bacterial meningitis | url = | journal = Neurology | volume = 73 | issue = 13| pages = 1026–32 | pmid = 19786694 }}</ref> On the other hand, conditions such as [[stress (medicine)|chronic stress]] and [[aging]] can result in a decreased neuronal proliferation.<ref>{{cite journal |author=Lee AL, Ogle WO, Sapolsky RM |title=Stress and depression: possible links to neuron death in the hippocampus |journal=Bipolar Disord. |volume=4 |issue=2 |pages=117–28 |year=2002 |month=April |pmid=12071509 |doi= 10.1034/j.1399-5618.2002.01144.x|url=http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=1398-5647&date=2002&volume=4&issue=2&spage=117}}</ref><ref>{{cite journal |author=Sheline YI, Gado MH, Kraemer HC |title=Untreated depression and hippocampal volume loss |journal=Am J Psychiatry. |volume=160 |issue=8 |pages=1516–8 |year=2003 |month=August |pmid=12900317 |doi= 10.1176/appi.ajp.160.8.1516|url=http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=12900317}}</ref><ref>{{cite pmid|16224541}}</ref> |
|||
Circulating factors within the blood may reduce neurogenesis. In healthy aging humans, the plasma and cerebrospinal fluid levels of certain chemokines are elevated. In a mouse model, plasma levels of these chemokines correlate with reduced neurogenesis, suggesting that neurogenesis may be modulated by certain global age-dependent systemic changes. These chemokines include [[CCL11]], [[CCL2]] and [[CCL12]], which are highly localized on mouse and human chromosomes, implicating a genetic locus in aging.<ref>{{Cite journal |
|||
| author = [[Saul A. Villeda]], [[Jian Luo]], [[Kira I. Mosher]], [[Bende Zou]], [[Markus Britschgi]], [[Gregor Bieri]], [[Trisha M. Stan]], [[Nina Fainberg]], [[Zhaoqing Ding]], [[Alexander Eggel]], [[Kurt M. Lucin]], [[Eva Czirr]], [[Jeong-Soo Park]], [[Sebastien Couillard-Despres]], [[Ludwig Aigner]], [[Ge Li]], [[Elaine R. Peskind]], [[Jeffrey A. Kaye]], [[Joseph F. Quinn]], [[Douglas R. Galasko]], [[Xinmin S. Xie]], [[Thomas A. Rando]] & [[Tony Wyss-Coray]] |
|||
| title = The ageing systemic milieu negatively regulates neurogenesis and cognitive function |
|||
| journal = [[Nature]] |
|||
| volume = 477 |
|||
| issue = 7362 |
|||
| pages = 90–94 |
|||
| year = 2011 |
|||
| month = September |
|||
| doi = 10.1038/nature10357 |
|||
| pmid = 21886162 |
|||
}}</ref> |
|||
==Adult neural stem cells== |
|||
{{main|neural stem cell}} |
|||
Neural [[stem cell]]s (NSCs) are the self-renewing, [[multipotency|multipotent]] cells that generate the main [[phenotypes]] of the [[nervous system]]. |
|||
==Effects of Marijuana== |
|||
Some studies have shown that use of cannabis results in the growth of new nerve cells in the hippocampus from both embryonic and adult stem cells. In 2005 a clinical study of rats at the University of Saskatchewan showed regeneration of nerve cells in the hippocampus.<ref>{{cite journal |url=http://www.jci.org/articles/view/25509 |title=Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects |author=Wen Jiang; Yun Zhang; Lan Xiao; Jamie Van Cleemput; Shao-Ping Ji; Guang Bai; Xia Zhang |journal=[[Journal of Clinical Investigation]] |volume=115 |issue=11 |pages=3104–16 |date=2005-11-01 |doi=10.1172/JCI25509 |pmc=1253627 |accessdate=2011-03-02 |pmid=16224541}}</ref> Studies have shown that a synthetic drug resembling THC, the main psychoactive ingredient in marijuana, provides some protection against brain inflammation, which might result in better memory at an older age. This is due to receptors in the system that can also influence the production of new neurons (http://www.osu.edu/news/newsitem2227) Nonetheless, a study directed at Rutgers University demonstrated how synchronization of action potentials in the hippocampus of rats was altered after THC administration. Lack of synchronization resulted in impaired performance in a standard test of memory. (http://www.physorg.com/news84048508.html) Moreover, contrary to popular belief, animal studies have revealed that marijuana could provoke fits.(http://www.livescience.com/1134-marijuana-impairs-memory.html) |
|||
==See also== |
|||
*[[Neural development]] |
|||
*[[Neuroplasticity]] |
|||
==References== |
|||
{{reflist|2}} |
|||
;Notes |
|||
{{refbegin}} |
|||
* Aimone JB, Jessberger S, and Gage FH (2007) Adult Neurogenesis. Scholarpedia, p. 8739 |
|||
*{{cite journal |author=Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E |title=Hippocampal neurogenesis in adult Old World primates |journal=Proc Natl Acad Sci U S A. |volume=96 |issue=9 |pages=5263–7 |year=1999 |month=April |pmid=10220454 |pmc=21852 |doi= 10.1073/pnas.96.9.5263|url=http://www.pnas.org/cgi/pmidlookup?view=long&pmid=10220454 |bibcode=1999PNAS...96.5263G}} |
|||
*{{cite journal |author=Gould E, Reeves AJ, Graziano MS, Gross CG |title=Neurogenesis in the neocortex of adult primates |journal=Science |volume=286 |issue=5439 |pages=548–52 |year=1999 |month=October |pmid=10521353 |doi= 10.1126/science.286.5439.548|url=http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=10521353}} |
|||
*{{cite journal |author=Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ |title=Learning enhances adult neurogenesis in the hippocampal formation |journal=Nat Neurosci. |volume=2 |issue=3 |pages=260–5 |year=1999 |month=March |pmid=10195219 |doi=10.1038/6365 |url=}} |
|||
*{{cite journal |author=Moghadam KS, Chen A, Heathcote RD |title=Establishment of a ventral cell fate in the spinal cord |journal=Dev. Dyn. |volume=227 |issue=4 |pages=552–62 |year=2003 |month=August |pmid=12889064 |doi=10.1002/dvdy.10340 |url=}} |
|||
*{{cite journal |author=Rakic P |title=Neurogenesis in adult primate neocortex: an evaluation of the evidence |journal=Nat Rev Neurosci. |volume=3 |issue=1 |pages=65–71 |year=2002 |month=January |pmid=11823806 |doi=10.1038/nrn700 |url=}} |
|||
* Rolls, E.T & Treves, A. (1998). Neural Networks and Brain Function. Oxford: OUP. ISBN 0-19-852432-3. |
|||
*{{cite journal |author=Santarelli L, Saxe M, Gross C, ''et al.'' |title=Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants |journal=Science |volume=301 |issue=5634 |pages=805–9 |year=2003 |month=August |pmid=12907793 |doi=10.1126/science.1083328 |url=|bibcode = 2003Sci...301..805S }} |
|||
*{{cite journal |author=Schloesser RJ, Manji HK, Martinowich K |title=Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. |journal=Neuroreport |volume=20 |issue=6 |pages=553–7 |year=2009 |month=April |pmid=19322118 |pmc=2693911 |doi=10.1097/WNR.0b013e3283293e59 |url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0959-4965&volume=20&issue=6&spage=553}} |
|||
* Shankle, WR, Rafii, MS, Landing, BH, and Fallon, JH (1999) Approximate doubling of the numbers of neurons in the postnatal human cortex and in 35 specific cytoarchitectonic areas from birth to 72 months. Pediatric and Developmental Pathology 2:244-259. |
|||
*{{cite journal |author=Zhao M, Momma S, Delfani K, ''et al.'' |title=Evidence for neurogenesis in the adult mammalian substantia nigra |journal=Proc Natl Acad Sci U S A. |volume=100 |issue=13 |pages=7925–30 |year=2003 |month=June |pmid=12792021 |pmc=164689 |doi=10.1073/pnas.1131955100 |url= |bibcode=2003PNAS..100.7925Z}} |
|||
*[http://publishing.royalsociety.org/brain-repair Dedicated issue of ''Philosophical Transactions B'' on Stem Cells and Brain Repair. Some articles are freely available.] |
|||
{{refend}} |
|||
==External links== |
==External links== |
||
*[http://www.wellesley.edu/Biology/Concepts/Html/neurogenesishow.html Concise |
* [http://www.wellesley.edu/Biology/Concepts/Html/neurogenesishow.html Concise Introduction to Neurogenesis] from [[Wellesley College]] |
||
*[http://sites.lafayette.edu/neur401-sp10/ Comprehensive website on |
*[http://sites.lafayette.edu/neur401-sp10/2010/05/04/welcome-to-our-neurogenesis-website/- Comprehensive website on Neurogenesis] from [[Lafayette College]] |
||
*[http://neurondevelopment.org/adult-neurogenesis Early literature on adult neurogenesis] |
*[http://neurondevelopment.org/adult-neurogenesis Early literature on adult neurogenesis] |
||
*[http://www.acnp.org/Docs/G5/CH8_109-118.pdf Neurogenesis in adult brain - Fred H. Gage and Henriette van Praag] |
*[http://www.acnp.org/Docs/G5/CH8_109-118.pdf Neurogenesis in adult brain - Fred H. Gage and Henriette van Praag] |
||
Revision as of 18:57, 24 October 2011
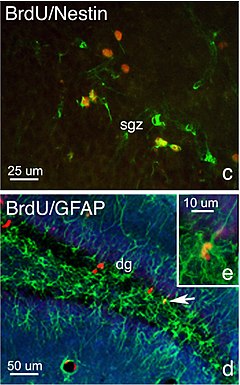
Neurogenesis (birth of neurons) is the process by which neurons are generated from neural stem and progenitor cells. Most active during pre-natal development, neurogenesis is responsible for populating the growing brain with neurons. Recently neurogenesis was shown to continue in several small parts of the brain of mammals: the hippocampus and the subventricular zone. Studies have indicated that hormones, such as testosterone in vertebrates and ecdysone in invertebrates, have an influence on the rate of neurogenesis.
Occurrence in adults

New neurons are continually born throughout adulthood in predominantly two regions of the brain:
- The subventricular zone (SVZ) lining the lateral ventricles, where neural stem cells and progenitor generate new neurons (Neuroblast) that migrate to the olfactory bulb via the rostral migratory stream
- The subgranular zone (SGZ), part of the dentate gyrus of hippocampus.
Many of the newborn cells die shortly after they are born, but a number of them become functionally integrated into the surrounding brain tissue.
Adult neurogenesis is an example of a long-held scientific theory being overturned. Early neuroanatomists, including Santiago Ramon y Cajal, considered the nervous system fixed and incapable of regeneration. The first evidence of adult mammalian neurogenesis in the cerebral cortex was presented by Joseph Altman in 1962,[3] followed by a demonstration of adult neurogenesis in the dentate gyrus of the hippocampus in 1963.[4] In 1969, Joseph Altman discovered and named the rostral migratory stream as the source of adult generated granule cell neurons in the olfactory bulb.[5] Up until the 1980s, the scientific community ignored these findings despite use of the most direct method of demonstrating cell proliferation in the early studies, i. e. 3H-thymidine autoradiography. By that time, Shirley Bayer [6][7] (and Michael Kaplan) again showed that adult neurogenesis exists in mammals (rats), and Nottebohm showed the same phenomenon in birds[8] sparking renewed interest in the topic. Studies in the 1990s[9][10] finally put research on adult neurogenesis into a mainstream pursuit. Also in the early 1990s hippocampal neurogenesis was demonstrated in non-human primates and humans.[11][12] More recently, neurogenesis in the cerebellum of adult rabbits has also been characterized.[13] Further, some authors (particularly Elizabeth Gould) have suggested that adult neurogenesis may also occur in regions within the brain not generally associated with neurogenesis including the neocortex.[14][15][16] However, others[17] have questioned the scientific evidence of these findings, arguing that the new cells may be of glial origin.
Role in learning
The functional relevance of adult neurogenesis is uncertain,[18] but there is some evidence that hippocampal adult neurogenesis is important for learning and memory.[19] Multiple mechanisms for the relationship between increased neurogenesis and improved cognition have been suggested, including computational theories to demonstrate that new neurons increase memory capacity,[20] reduce interference between memories,[21] or add information about time to memories.[22] Experiments aimed at ablating neurogenesis have proven inconclusive, but several studies have proposed neurogenic-dependence in some types of learning,[23] and others seeing no effect.[24] Studies have demonstrated that the act of learning itself is associated with increased neuronal survival.[25] However, the overall findings that adult neurogenesis is important for any kind of learning are equivocal.
Effects of stress
Adult-born neurons appear to have a role in the regulation of stress. Studies have linked neurogenesis to the beneficial actions of specific antidepressants, suggesting a connection between decreased hippocampal neurogenesis and depression.[26][27] In a subsequent paper, scientists demonstrated that the behavioral benefits of antidepressant administration in mice is reversed when neurogenesis is prevented with x-irradiation techniques.[28] In fact, new-born neurons are more excitable than older neurons due to a differential expression of GABA receptors.[citation needed] A plausible model, therefore, is that these neurons augment the role of the hippocampus in the negative feedback mechanism of the HPA-axis (physiological stress) and perhaps in inhibiting the amygdala (the region of brain responsible for fearful responses to stimuli).[vague] Indeed, suppression of adult neurogenesis can lead to an increased HPA-axis stress response in mildly stressful situations.[29] This is consistent with numerous findings linking stress-relieving activities (learning, exposure to a new yet benign environment, and exercise) to increased levels of neurogenesis, as well as the observation that animals exposed to physiological stress (cortisol) or psychological stress (e.g. isolation) show markedly decreased levels of new-born neurons. Strikingly, the elevation of newborn neurons by antidepressants improves, under chronic stress conditions, the hippocampal control on the stress response (including the activity of the HPA axis and of stress-integrative brain nuclei), then leading to recovery; without newborn neurons, antidepressants are unable to restore the regulation of the stress response and recovery becomes impossible.[30]
Some studies have hypothesized that learning and memory are linked to depression, and that neurogenesis may promote neuroplasticity. One study proposes that mood may be regulated, at a base level, by plasticity, and thus not chemistry. Accordingly, the effects of antidepressant treatment would only be secondary to change in plasticity.[31]
Effects of sleep reduction
One study has linked lack of sleep to a reduction in rodent hippocampal neurogenesis. The proposed mechanism for the observed decrease was increased levels of glucocorticoids. It was shown that two weeks of sleep deprivation acted as a neurogenesis-inhibitor, which was reversed after return of normal sleep and even shifted to a temporary increase in normal cell proliferation.[32] More precisely, when levels of corticosterone are elevated, sleep deprivation inhibits this process. Nonetheless, normal levels of neurogenesis after chronic sleep deprivation return after 2 weeks, with a temporary increase of neurogenesis. (http://www.pnas.org/content/103/50/19170.full)
Possible use in treating Parkinson's disease
Parkinson's disease is a neurodegenerative disorder characterized by a progressive loss of dopaminergic neurons in the nigrostriatal projection. Transplantation of fetal dopaminergic precursor cells has paved the way for the possibility of a cell replacement therapy that could ameliorate clinical symptoms in affected patients.[33] Recent years have provided evidence for the existence of neural stem cells with the potential to produce new neurons, particularly of a dopaminergic phenotype, in the adult mammalian brain.[34][35][36] Experimental depletion of dopamine in rodents decreases precursor cell proliferation in both the subependymal zone and the subgranular zone.[37] Proliferation is restored completely by a selective agonist of D2-like (D2L) receptors.[37] Neural stem cells have been identified in the neurogenic brain regions, where neurogenesis is constitutively ongoing, but also in the non-neurogenic zones, such as the midbrain and the striatum, where neurogenesis is not thought to occur under normal physiological conditions.[33] A detailed understanding of the factors governing adult neural stem cells in vivo may ultimately lead to elegant cell therapies for neurodegenerative disorders such as Parkinson's disease by mobilizing autologous endogenous neural stem cells to replace degenerated neurons.[33]
Role in behavioral sensitization
Reinforcing drugs such as amphetamines and opiates induce behavioral sensitization upon repeated administration by inducing dopaminergic neurogenesis in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc).[38][39][40][41][42] This occurs through activation of dopamine receptors in these areas which produces glutamate release and subsequent elevation of local basic fibroblast growth factor (bFGF) concentrations.[38][39][40][41][42] The consequences of these actions are potentiated reward responses and therefore increased drug cravings and consumption which underlie abuse and addiction. Whether these mechanisms could be exploited for the purpose of enhancing basal hedonic tone is unknown.
Effects of exercise
Scientists have shown that physical activity in the form of voluntary exercise results in an increase in the number of newborn neurons in the hippocampus of aging mice. The same study demonstrates an enhancement in learning of the "runner" (physically active) mice.[43][44] Another research, nonetheless, demonstrated that mice exercising that did not produce beta-endorphin, a mood-elevating hormone, had no change in neurogenesis. Yet, mice that did produce this hormone, along with exercising, exhibited an increase in newborn cells and their rate of survival ( http://www.sfn.org/index.aspx?pagename=brainbriefings_adult_neurogenesis). While the association between exercise-mediated neurogenesis and enhancement of learning remains unclear, this study could have strong implications in the fields of aging and/or [Alzheimer's disease]].
Changes in old age
Neurogenesis is substantially reduced in the hippocampus of aged animals, raising the possibility that it may be linked to age-related declines in hippocampal function. Given that neurogenesis occurs throughout life, it might be expected that the hippocampus would steadily increase in size during adulthood, and that therefore the number of granule cells would be increased in aged animals. However, this is not the case, indicating that proliferation is balanced by cell death. Thus, it is not the addition of new neurons into the hippocampus that seems to be linked to hippocampal functions, but rather the rate of turnover of granule cells.[45].
Alzheimer's disease
Allopregnanolone, a neurosteroid, aids the continued neurogenesis in the brain. Levels of allopregnanolone in the brain decline in old age and Alzheimer's disease.[46] Allopregnanolone has been shown through reversing impairment of neurogenesis to reverse the cognitive deficits in a mouse model of Alzheimer's disease.[47]
Regulation
Many factors may affect the rate of hippocampal neurogenesis. Exercise and an enriched environment have been shown to promote the survival of neurons and the successful integration of newborn cells into the existing hippocampus.,[43][48][49][50] Another factor is central nervous system injury since neurogenesis occurs after cerebral ischemia,[51] epileptic seizures,[52] and bacterial meningitis.[53] On the other hand, conditions such as chronic stress and aging can result in a decreased neuronal proliferation.[54][55][56]
Circulating factors within the blood may reduce neurogenesis. In healthy aging humans, the plasma and cerebrospinal fluid levels of certain chemokines are elevated. In a mouse model, plasma levels of these chemokines correlate with reduced neurogenesis, suggesting that neurogenesis may be modulated by certain global age-dependent systemic changes. These chemokines include CCL11, CCL2 and CCL12, which are highly localized on mouse and human chromosomes, implicating a genetic locus in aging.[57]
Adult neural stem cells
Neural stem cells (NSCs) are the self-renewing, multipotent cells that generate the main phenotypes of the nervous system.
Effects of Marijuana
Some studies have shown that use of cannabis results in the growth of new nerve cells in the hippocampus from both embryonic and adult stem cells. In 2005 a clinical study of rats at the University of Saskatchewan showed regeneration of nerve cells in the hippocampus.[58] Studies have shown that a synthetic drug resembling THC, the main psychoactive ingredient in marijuana, provides some protection against brain inflammation, which might result in better memory at an older age. This is due to receptors in the system that can also influence the production of new neurons (http://www.osu.edu/news/newsitem2227) Nonetheless, a study directed at Rutgers University demonstrated how synchronization of action potentials in the hippocampus of rats was altered after THC administration. Lack of synchronization resulted in impaired performance in a standard test of memory. (http://www.physorg.com/news84048508.html) Moreover, contrary to popular belief, animal studies have revealed that marijuana could provoke fits.(http://www.livescience.com/1134-marijuana-impairs-memory.html)
See also
References
- ^ Faiz M, Acarin L, Castellano B, Gonzalez B (2005). "Proliferation dynamics of germinative zone cells in the intact and excitotoxically lesioned postnatal rat brain". BMC Neurosci. 6: 26. doi:10.1186/1471-2202-6-26. PMC 1087489. PMID 15826306.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Oomen CA, Girardi CE, Cahyadi R, ''et al.'' (2009). Baune, Bernhard (ed.). "Opposite effects of early maternal deprivation on neurogenesis in male versus female rats". PLoS ONE. 4 (1): e3675. Bibcode:2009PLoSO...4.3675O. doi:10.1371/journal.pone.0003675. PMC 2629844. PMID 19180242.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 13860748, please use {{cite journal}} with
|pmid=13860748instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 14012334, please use {{cite journal}} with
|pmid=14012334instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 5361244, please use {{cite journal}} with
|pmid=5361244instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7079742, please use {{cite journal}} with
|pmid=7079742instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7095040, please use {{cite journal}} with
|pmid=7095040instead. - ^ Goldman SA, Nottebohm F (1983). "Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain". Proc Natl Acad Sci U S A. 80 (8): 2390–4. Bibcode:1983PNAS...80.2390G. doi:10.1073/pnas.80.8.2390. PMC 393826. PMID 6572982.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 1553558, please use {{cite journal}} with
|pmid=1553558instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7605059, please use {{cite journal}} with
|pmid=7605059instead. - ^ Eriksson PS, Perfilieva E, Björk-Eriksson T; et al. (1998). "Neurogenesis in the adult human hippocampus". Nat Med. 4 (11): 1313–7. doi:10.1038/3305. PMID 9809557.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10220454, please use {{cite journal}} with
|pmid=10220454instead. - ^ Ponti G, Peretto B, Bonfanti L (2008). Reh, Thomas A. (ed.). "Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits". PLoS ONE. 3 (6): e2366. Bibcode:2008PLoSO...3.2366P. doi:10.1371/journal.pone.0002366. PMC 2396292. PMID 18523645.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10521353, please use {{cite journal}} with
|pmid=10521353instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12792021, please use {{cite journal}} with
|pmid=12792021instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10191348, please use {{cite journal}} with
|pmid=10191348instead. - ^ Rakic P (2002). "Adult neurogenesis in mammals: an identity crisis". J. Neurosci. 22 (3): 614–8. PMID 11826088.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kempermann G, Wiskott L, Gage FH (2004). "Functional significance of adult neurogenesis". Curr Opin Neurobiol. 14 (2): 186–91. doi:10.1016/j.conb.2004.03.001. PMID 15082323.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ G. Neves, G (2008). "Synaptic plasticity, memory and the hippocampus: A neural network approach to causality". Nature Reviews Neuroscience. 9 (1): 65–75. doi:10.1038/nrn2303. PMID 18094707.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Becker S (2005). "A computational principle for hippocampal learning and neurogenesis". Hippocampus. 15 (6): 722–38. doi:10.1002/hipo.20095. PMID 15986407.
- ^ Wiskott L, Rasch MJ, Kempermann G (2006). "A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus". Hippocampus. 16 (3): 329–43. doi:10.1002/hipo.20167. PMID 16435309.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Aimone JB, Wiles J, Gage FH (2006). "Potential role for adult neurogenesis in the encoding of time in new memories". Nat Neurosci. 9 (6): 723–7. doi:10.1038/nn1707. PMID 16732202.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E (2002). "Neurogenesis may relate to some but not all types of hippocampal-dependent learning". Hippocampus. 12 (5): 578–84. doi:10.1002/hipo.10103. PMID 12440573.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Meshi D, Drew MR, Saxe M; et al. (2006). "Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment". Nat Neurosci. 9 (6): 729–31. doi:10.1038/nn1696. PMID 16648847.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10195219, please use {{cite journal}} with
|pmid= 10195219instead. - ^ Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000). "Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus". J Neurosci. 20 (24): 9104–10. PMID 11124987.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Manev H, Uz T, Smalheiser NR, Manev R (2001). "Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro". Eur J Pharmacol. 411 (1–2): 67–70. doi:10.1016/S0014-2999(00)00904-3. PMID 11137860.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Santarelli L, Saxe M, Gross C; et al. (2003). "Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants". Science. 301 (5634): 805–9. Bibcode:2003Sci...301..805S. doi:10.1126/science.1083328. PMID 12907793.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schloesser RJ, Manji HK, Martinowich K (2009). "Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response". Neuroreport. 20 (6): 553–7. doi:10.1097/WNR.0b013e3283293e59. PMC 2693911. PMID 19322118.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Surget A, Tanti A, Leonardo ED; et al. (2011). "Antidepressants recruit new neurons to improve stress response regulation". Molecular Psychiatry (advance online publication). doi:10.1038/mp.2011.48. PMID 21537331.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Castrén E (2005). "Is mood chemistry?". Nat Rev Neurosci. 6 (3): 241–6. doi:10.1038/nrn1629. PMID 15738959.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Mirescu C, Peters JD, Noiman L, Gould E (2006). "Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids". Proc Natl Acad Sci U S A. 103 (50): 19170–5. Bibcode:2006PNAS..10319170M. doi:10.1073/pnas.0608644103. PMC 1748194. PMID 17135354.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Arias-Carrión O, Freundlieb N, Oertel WH, Höglinger GU (2007). "Adult neurogenesis and Parkinson's disease". CNS Neurol Disord Drug Targets. 6 (5): 326–35. doi:10.2174/187152707783220875. PMID 18045161.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Fallon J, Reid S, Kinyamu R; et al. (2000). "In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain". Proc Natl Acad Sci U S A. 97 (26): 14686–91. Bibcode:2000PNAS...9714686F. doi:10.1073/pnas.97.26.14686. PMC 18979. PMID 11121069.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Arias-Carrión O, Verdugo-Díaz L, Feria-Velasco A; et al. (2004). "Neurogenesis in the subventricular zone following transcranial magnetic field stimulation and nigrostriatal lesions". J Neurosci Res. 78 (1): 16–28. doi:10.1002/jnr.20235. PMID 15372495.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Arias-Carrión O, Hernández-López S, Ibañez-Sandoval O, Bargas J, Hernández-Cruz A, Drucker-Colín R (2006). "Neuronal precursors within the adult rat subventricular zone differentiate into dopaminergic neurons after substantia nigra lesion and chromaffin cell transplant". J Neurosci Res. 84 (7): 1425–37. doi:10.1002/jnr.21068. PMID 17006899.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Höglinger GU, Rizk P, Muriel MP; et al. (2004). "Dopamine depletion impairs precursor cell proliferation in Parkinson disease". Nat Neurosci. 7 (7): 726–35. doi:10.1038/nn1265. PMID 15195095.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Flores C, Rodaros D, Stewart J (1998). "Long-lasting induction of astrocytic basic fibroblast growth factor by repeated injections of amphetamine: blockade by concurrent treatment with a glutamate antagonist". Journal of Neuroscience. 18 (22): 9547–55. PMID 9801391.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Flores C, Stewart J (2000). "Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat". Psychopharmacology. 151 (2–3): 152–65. doi:10.1007/s002130000417. PMID 10972461.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Flores C, Samaha AN, Stewart J (2000). "Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine". Journal of Neuroscience. 20 (2): RC55. PMID 10632621.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Pierce RC, Bari AA (2001). "The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity". Reviews in the Neurosciences. 12 (2): 95–110. PMID 11392459.
- ^ a b Mueller D, Chapman CA, Stewart J (2006). "Amphetamine induces dendritic growth in ventral tegmental area dopaminergic neurons in vivo via basic fibroblast growth factor". Neuroscience. 137 (3): 727–35. doi:10.1016/j.neuroscience.2005.09.038. PMID 16338078.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS (2005). "Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice". Cell. 120 (5): 701–13. doi:10.1016/j.cell.2005.01.015. PMID 15766532.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ van Praag H, Shubert T, Zhao C, Gage FH (2005). "Exercise enhances learning and hippocampal neurogenesis in aged mice". J. Neurosci. 25 (38): 8680–5. doi:10.1523/JNEUROSCI.1731-05.2005. PMC 1360197. PMID 16177036.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ von Bohlen und Halbach O (2010). "Involvement of BDNF in age-dependent alterations in the hippocampus". Front Aging Neurosci. 2. doi:10.3389/fnagi.2010.00036. PMC 2952461. PMID 20941325.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Marx CE, Trost WT, Shampine LJ; et al. (2006). "The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease". Biol. Psychiatry. 60 (12): 1287–94. doi:10.1016/j.biopsych.2006.06.017. PMID 16997284.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD (2010). "Allopregnanolone reverses neuron and cognitive deficits in a mouse model of Alzheimer's disease" (PDF). Proc Natl Acad Sci U S A. 107 (14): 6498–6503. Bibcode:2010PNAS..107.6498W. doi:10.1073/pnas.1001422107. PMC 2851948. PMID 20231471.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16177036, please use {{cite journal}} with
|pmid=16177036instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10195220, please use {{cite journal}} with
|pmid=10195220instead. - ^ Bjørnebekk A, Mathé AA, Brené S (2005). "The antidepressant effect of running is associated with increased hippocampal cell proliferation". Int J Neuropsychopharmacol. 8 (3): 357–68. doi:10.1017/S1461145705005122. PMID 15769301.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jin K, Wang X, Xie L; et al. (2006). "Evidence for stroke-induced neurogenesis in the human brain". Proc. Natl. Acad. Sci. U.S.A. 103 (35): 13198–202. Bibcode:2006PNAS..10313198J. doi:10.1073/pnas.0603512103. PMC 1559776. PMID 16924107.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Parent JM; Elliott, RC; Pleasure, SJ; Barbaro, NM; Lowenstein, DH (2006). "Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy". Ann Neurol. 59 (1): 81–91. doi:10.1002/ana.20699. PMID 16261566.
- ^ Gerber J, Tauber SC, Armbrecht I, Schmidt H, Brück W, Nau R (2009). "Increased neuronal proliferation in human bacterial meningitis". Neurology. 73 (13): 1026–32. doi:10.1212/WNL.0b013e3181b9c892. PMID 19786694.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lee AL, Ogle WO, Sapolsky RM (2002). "Stress and depression: possible links to neuron death in the hippocampus". Bipolar Disord. 4 (2): 117–28. doi:10.1034/j.1399-5618.2002.01144.x. PMID 12071509.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sheline YI, Gado MH, Kraemer HC (2003). "Untreated depression and hippocampal volume loss". Am J Psychiatry. 160 (8): 1516–8. doi:10.1176/appi.ajp.160.8.1516. PMID 12900317.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16224541, please use {{cite journal}} with
|pmid=16224541instead. - ^ Saul A. Villeda, Jian Luo, Kira I. Mosher, Bende Zou, Markus Britschgi, Gregor Bieri, Trisha M. Stan, Nina Fainberg, Zhaoqing Ding, Alexander Eggel, Kurt M. Lucin, Eva Czirr, Jeong-Soo Park, Sebastien Couillard-Despres, Ludwig Aigner, Ge Li, Elaine R. Peskind, Jeffrey A. Kaye, Joseph F. Quinn, Douglas R. Galasko, Xinmin S. Xie, Thomas A. Rando & Tony Wyss-Coray (2011). "The ageing systemic milieu negatively regulates neurogenesis and cognitive function". Nature. 477 (7362): 90–94. doi:10.1038/nature10357. PMID 21886162.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wen Jiang; Yun Zhang; Lan Xiao; Jamie Van Cleemput; Shao-Ping Ji; Guang Bai; Xia Zhang (2005-11-01). "Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects". Journal of Clinical Investigation. 115 (11): 3104–16. doi:10.1172/JCI25509. PMC 1253627. PMID 16224541. Retrieved 2011-03-02.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- Notes
- Aimone JB, Jessberger S, and Gage FH (2007) Adult Neurogenesis. Scholarpedia, p. 8739
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E (1999). "Hippocampal neurogenesis in adult Old World primates". Proc Natl Acad Sci U S A. 96 (9): 5263–7. Bibcode:1999PNAS...96.5263G. doi:10.1073/pnas.96.9.5263. PMC 21852. PMID 10220454.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Gould E, Reeves AJ, Graziano MS, Gross CG (1999). "Neurogenesis in the neocortex of adult primates". Science. 286 (5439): 548–52. doi:10.1126/science.286.5439.548. PMID 10521353.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999). "Learning enhances adult neurogenesis in the hippocampal formation". Nat Neurosci. 2 (3): 260–5. doi:10.1038/6365. PMID 10195219.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Moghadam KS, Chen A, Heathcote RD (2003). "Establishment of a ventral cell fate in the spinal cord". Dev. Dyn. 227 (4): 552–62. doi:10.1002/dvdy.10340. PMID 12889064.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Rakic P (2002). "Neurogenesis in adult primate neocortex: an evaluation of the evidence". Nat Rev Neurosci. 3 (1): 65–71. doi:10.1038/nrn700. PMID 11823806.
{{cite journal}}: Unknown parameter|month=ignored (help)
- Rolls, E.T & Treves, A. (1998). Neural Networks and Brain Function. Oxford: OUP. ISBN 0-19-852432-3.
- Santarelli L, Saxe M, Gross C; et al. (2003). "Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants". Science. 301 (5634): 805–9. Bibcode:2003Sci...301..805S. doi:10.1126/science.1083328. PMID 12907793.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Schloesser RJ, Manji HK, Martinowich K (2009). "Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response". Neuroreport. 20 (6): 553–7. doi:10.1097/WNR.0b013e3283293e59. PMC 2693911. PMID 19322118.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Shankle, WR, Rafii, MS, Landing, BH, and Fallon, JH (1999) Approximate doubling of the numbers of neurons in the postnatal human cortex and in 35 specific cytoarchitectonic areas from birth to 72 months. Pediatric and Developmental Pathology 2:244-259.
- Zhao M, Momma S, Delfani K; et al. (2003). "Evidence for neurogenesis in the adult mammalian substantia nigra". Proc Natl Acad Sci U S A. 100 (13): 7925–30. Bibcode:2003PNAS..100.7925Z. doi:10.1073/pnas.1131955100. PMC 164689. PMID 12792021.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Dedicated issue of Philosophical Transactions B on Stem Cells and Brain Repair. Some articles are freely available.
External links
- Concise Introduction to Neurogenesis from Wellesley College
- Comprehensive website on Neurogenesis from Lafayette College
- Early literature on adult neurogenesis
- Neurogenesis in adult brain - Fred H. Gage and Henriette van Praag
- "Neurogenesis and Parkinson´s disease"
- Scholarpedia Article on Adult Neurogenesis
- "TRENDS in Neurosciences, 10 October 2001 (Michael S. Kaplan MD, PhD)
- New York Times: Studies Find Brains Grow New Cells
- New Yorker: Rethinking the Brain - How the songs of canaries upset a fundamental principle of science
- The Neurogenesis Experiment - Article series on adult human neurogenesis
- Seed magazine: The Reinvention of the Self - A historical background on the field of neurogenesis and implications of this research
- BBC Radio 4: The Memory Experience - Use it or Lose it
- PBS: Changing Your Mind - Grow Your Own Brain
- New York Times "Play" Magazine - Lobes of Steel, Article on Exercise Promoting Neurogenesis
 Media related to neurogenesis at Wikimedia Commons
Media related to neurogenesis at Wikimedia Commons
