Atrial fibrillation: Difference between revisions
| Line 242: | Line 242: | ||
* [http://www.patient.co.uk/showdoc/23068682/ Atrial fibrillation] at patient.co.uk |
* [http://www.patient.co.uk/showdoc/23068682/ Atrial fibrillation] at patient.co.uk |
||
* [http://www.jr2.ox.ac.uk/bandolier/booth/booths/AF.html Bandolier] (evidence-based medicine) resource on atrial fibrillation |
* [http://www.jr2.ox.ac.uk/bandolier/booth/booths/AF.html Bandolier] (evidence-based medicine) resource on atrial fibrillation |
||
*[https://online.epocrates.com/u/29111/Chronic+atrial+fibrillation Atrial fibrillation] at [https://online.epocrates.com Epocrates Online] |
* [https://online.epocrates.com/u/29111/Chronic+atrial+fibrillation Atrial fibrillation] at [https://online.epocrates.com Epocrates Online] |
||
* [http://www.heartpatients.org/page/atrial-fibrillation Atrial fibrillation] at HeartPatients Foundation |
|||
{{Circulatory system pathology}} |
{{Circulatory system pathology}} |
||
Revision as of 08:38, 28 August 2010
| Atrial fibrillation | |
|---|---|
| Specialty | Cardiology |
Atrial fibrillation (AF or A-fib) is the most common cardiac arrhythmia (abnormal heart rhythm)[1] and involves the two upper chambers (atria) of the heart. Its name comes from the fibrillating (i.e., quivering) of the heart muscles of the atria, instead of a coordinated contraction. It can often be identified by taking a pulse and observing that the heartbeats don't occur at regular intervals. However, a stronger indicator of AF is the absence of P waves on an electrocardiogram (ECG or EKG), which are normally present when there is a coordinated atrial contraction at the beginning of each heart beat.[2] Risk increases with age, with 8% of people over 80 having AF.
In AF, the normal electrical impulses that are generated by the sinoatrial node are overwhelmed by disorganized electrical impulses that originate in the atria and pulmonary veins, leading to conduction of irregular impulses to the ventricles that generate the heartbeat. The result is an irregular heartbeat, which may occur in episodes lasting from minutes to weeks, or it could occur all the time for years. The natural tendency of AF is to become a chronic condition. Chronic AF leads to a small increase in the risk of death.[3][4]
Atrial fibrillation is often asymptomatic and is not in itself generally life-threatening, but it may result in palpitations, fainting, chest pain, or congestive heart failure. People with AF usually have a significantly increased risk of stroke (up to 7 times that of the general population). Stroke risk increases during AF because blood may pool and form clots in the poorly contracting atria and especially in the left atrial appendage (LAA).[5] The level of increased risk of stroke depends on the number of additional risk factors. If a person with AF has none, the risk of stroke is similar to that of the general population.[6] However, many people with AF do have additional risk factors and AF is a leading cause of stroke.[7]
Atrial fibrillation may be treated with medications which either slow the heart rate or revert the heart rhythm back to normal. Synchronized electrical cardioversion may also be used to convert AF to a normal heart rhythm. Surgical and catheter-based therapies may also be used to prevent recurrence of AF in certain individuals. People with AF are often given anticoagulants such as warfarin to protect them from stroke.
Classification
The American College of Cardiology (ACC), American Heart Association (AHA), and the European Society of Cardiology (ESC) recommend in their guidelines the following classification system based on simplicity and clinical relevance.[8]
| AF Category | Defining Characteristics |
|---|---|
| First detected | only one diagnosed episode |
| Paroxysmal | recurrent episodes that self-terminate in less than 7 days. |
| Persistent | recurrent episodes that last more than 7 days |
| Permanent | an ongoing long-term episode |
All atrial fibrillation patients are initially in the category called first detected AF. These patients may or may not have had previous undetected episodes. If a first detected episode self-terminates in less than 7 days and then another episode begins later on, the case has moved into the category of paroxysmal AF. Although patients in this category have episodes lasting up to 7 days, in most cases of paroxysmal AF the episodes will self-terminate in less than 24 hours. If instead the episode lasts for more than 7 days, it is unlikely to self-terminate[9] and it is called persistent AF. In this case, the episode may be terminated by cardioversion. If cardioversion is unsuccessful or it is not attempted, and the episode is ongoing for a long time (e.g. a year or more), the patient's AF is called permanent.
Episodes that last less than 30 seconds are not considered in this classification system. Also, this system does not apply to cases where the AF is a secondary condition that occurs in the setting of a primary condition that may be the cause of the AF.
Using this classification system, it's not always clear what an AF case should be called. For example, a case may fit into the paroxysmal AF category some of the time, while other times it may have the characteristics of persistent AF. One may be able to decide which category is more appropriate by determining which one occurs most often in the case under consideration.
In addition to the above four AF categories, which are mainly defined by episode timing and termination, the ACC/AHA/ESC guidelines describe additional AF categories in terms of other characteristics of the patient.[8]
- Lone atrial fibrillation (LAF) - absence of clinical or echocardiographic findings of other cardiovascular disease (including hypertension), related pulmonary disease, or cardiac abnormalities such as enlargement of the left atrium, and age under 60 years
- Nonvalvular AF - absence of rheumatic mitral valve disease, a prosthetic heart valve, or mitral valve repair
- Secondary AF - occurs in the setting of a primary condition which may be the cause of the AF, such as acute myocardial infarction, cardiac surgery, pericarditis, myocarditis, hyperthyroidism, pulmonary embolism, pneumonia, or other acute pulmonary disease
Signs and symptoms
Atrial fibrillation is usually accompanied by symptoms related to a rapid heart rate. Rapid and irregular heart rates may be perceived as palpitations, exercise intolerance, and occasionally produce angina (if the rate is faster and puts the heart under strain) and congestive symptoms of shortness of breath or edema. Sometimes the arrhythmia will be identified only with the onset of a stroke or a transient ischemic attack (TIA). It is not uncommon for a patient to first become aware of AF from a routine physical examination or ECG, as it may be asymptomatic in many cases.[8]
As most cases of atrial fibrillation are secondary to other medical problems, the presence of chest pain or angina, symptoms of hyperthyroidism (an overactive thyroid gland) such as weight loss and diarrhea, and symptoms suggestive of lung disease would indicate an underlying cause. A previous history of stroke or TIA, as well as hypertension (high blood pressure), diabetes, heart failure and rheumatic fever, may indicate whether someone with AF is at a higher risk of complications.[8]
Rapid heart rate
Presentation is similar to other forms of rapid heart rate (tachycardia), and in some cases may actually be asymptomatic. The patient may complain of palpitations or chest discomfort. The rapid heart rate may result in the heart being unable to provide adequate blood flow and oxygen delivery to the rest of the body.Therefore, common symptoms may include shortness of breath which often worsens with exertion (dyspnea on exertion), shortness of breath when lying flat (orthopnea), and sudden onset of shortness of breath during the night (paroxysmal nocturnal dyspnea), and may progress to swelling of the lower extremities (peripheral edema).Due to inadequate blood flow, patients may also complain of light-headedness, may feel like they are about to faint (presyncope), or may actually lose consciousness (syncope).
The patient may be in significant respiratory distress. Due to inadequate oxygen delivery, the patient may appear blue (cyanosis). By definition, the heart rate will be greater than 100 beats per minute. Blood pressure will be variable, and often difficult to measure as the beat-by-beat variability causes problems for most digital (oscillometric) non-invasive blood pressure monitors. It is most concerning if consistently lower than usual (hypotension). Respiratory rate will be increased in the presence of respiratory distress. Pulse oximetry may confirm the presence of hypoxia related to any precipitating factors such as pneumonia. Examination of the jugular veins may reveal elevated pressure (jugular venous distention). Lung exam may reveal rales or crackles, which are suggestive of pulmonary edema. Heart exam will reveal an irregular but rapid rhythm.
Diagnosis
The evaluation of atrial fibrillation involves diagnosis, determination of the etiology of the arrhythmia, and classification of the arrhythmia. A minimal evaluation should be performed in all individuals with AF. This includes a history and physical examination, ECG, transthoracic echocardiogram, and routine bloodwork. Certain individuals may benefit from an extended evaluation which may include an evaluation of the heart rate response to exercise, exercise stress testing, a chest x-ray, trans-esophageal echocardiography, and other studies.
If a patient presents with a sudden onset of severe symptoms other forms of tachyarrhythmia must be ruled-out, as some may be immediately life threatening, such as ventricular tachycardia. While most patients will be placed on continuous cardiorespiratory monitoring, an electrocardiogram (EKG) is essential for diagnosis.
Provoking causes should be sought out. A common cause of any tachycardia is dehydration, as well as other forms of hypovolemia. Acute coronary syndrome should be ruled out. Intercurrent illness such as pneumonia may be present.
Screening
Screening for atrial fibrillation is not generally performed, although a study of routine pulse checks or ECGs during routine office visits found that the annual rate of detection of AF in elderly patients improved from 1.04% to 1.63%; selection of patients for prophylactic anticoagulation would improve stroke risk in that age category.[10]
Routine primary care visit
This estimated sensitivity of the routine primary care visit is 64%. This low result probably reflects the pulse not being checked routinely or carefully.[10]
Minimal evaluation
The minimal evaluation of atrial fibrillation should generally be performed in all individuals with AF. The goal of this evaluation is to determine the general treatment regimen for the individual. If results of the general evaluation warrant it, further studies may then be performed.
History and physical examination
The history of the individual's atrial fibrillation episodes is probably the most important part of the evaluation. Distinctions should be made between those who are entirely asymptomatic when they are in AF (in which case the AF is found as an incidental finding on an ECG or physical examination) and those who have gross and obvious symptoms due to AF and can pinpoint whenever they go into AF or revert to sinus rhythm.
Routine bloodwork
While many cases of AF have no definite cause, it may be the result of various other problems (see below). Hence, renal function and electrolytes are routinely determined, as well as thyroid-stimulating hormone (commonly suppressed in hyperthyroidism and of relevance if amiodarone is administered for treatment) and a blood count.[8]
In acute-onset AF associated with chest pain, cardiac troponins or other markers of damage to the heart muscle may be ordered. Coagulation studies (INR/aPTT) are usually performed, as anticoagulant medication may be commenced.[8]
Electrocardiogram

Atrial fibrillation is diagnosed on an electrocardiogram (ECG), an investigation performed routinely whenever an irregular heart beat is suspected. Characteristic findings are the absence of P waves, with unorganized electrical activity in their place, and irregular R-R intervals due to irregular conduction of impulses to the ventricles.[8] However, irregular R-R intervals may be difficult to determine if the rate is extremely rapid.[11]
QRS complexes should be narrow, signifying that they are initiated by normal conduction of atrial electrical activity through the intraventricular conduction system. Wide QRS complexes are worrisome for ventricular tachycardia, although in cases where there is disease of the conduction system, wide complexes may be present in afib with rapid ventricular response.
When ECGs are used for screening, the SAFE trial found that electronic software, primary care physicians and the combination of the two had the following sensitivities and specificities:[12]:
- Interpreted by software: sensitivity = 83%, specificity = 99%
- Interpreted by a primary care physician: sensitivity = 80%, specificity = 92%
- Interpreted by a primary care physician with software: sensitivity = 92%, specificity = 91%
If paroxysmal AF is suspected but an ECG during an office visit only shows a regular rhythm, AF episodes may be detected and documented with the use of ambulatory Holter monitoring (e.g. for a day). If the episodes are too infrequent to be detected by Holter monitoring with reasonable probability, then the patient can be monitored for longer periods (e.g. a month) with an ambulatory event monitor.[8]
Echocardiography
A non-invasive transthoracic echocardiogram (TTE) is generally performed in newly diagnosed AF, as well as if there is a major change in the patient's clinical state. This ultrasound-based scan of the heart may help identify valvular heart disease (which may greatly increase the risk of stroke), left and right atrial size (which indicates likelihood that AF may become permanent), left ventricular size and function, peak right ventricular pressure (pulmonary hypertension), presence of left atrial thrombus (low sensivity), presence of left ventricular hypertrophy and pericardial disease.[8]
Significant enlargement of both the left and right atria is associated with long-standing atrial fibrillation and, if noted at the initial presentation of atrial fibrillation, suggests that the atrial fibrillation is likely to be of a longer duration than the individual's symptoms.
Extended evaluation
An extended evaluation is generally not necessary in most individuals with atrial fibrillation, and is only performed if abnormalities are noted in the limited evaluation, if a reversible cause of the atrial fibrillation is suggested, or if further evaluation may change the treatment course.
Chest X-ray
A chest X-ray is generally only performed if a pulmonary cause of atrial fibrillation is suggested, or if other cardiac conditions are suspected (particularly congestive heart failure.) This may reveal an underlying problem in the lungs or the blood vessels in the chest.[8] In particular, if an underlying pneumonia is suggested, then treatment of the pneumonia may cause the atrial fibrillation to terminate on its own.
Transesophageal echocardiogram
A normal echocardiography (transthoracic or TTE) has a low sensitivity for identifying thrombi (blood clots) in the heart. If this is suspected - e.g. when planning urgent electrical cardioversion - a transesophageal echocardiogram (TEE or TOE where British spelling is used) is preferred.[8]
The TEE has much better visualization of the left atrial appendage than transthoracic echocardiography. This structure, located in the left atrium, is the place where thrombus is formed in more than 90% of cases in non-valvular (or non-rheumatic) atrial fibrillation or flutter.[5] TEE has a high sensitivity for locating thrombi in this area[13] and can also detect sluggish bloodflow in this area that is suggestive of thrombus formation.[14]
If no thrombus is seen on TEE, the incidence of stroke, (immediately after cardioversion is performed), is very low.[citation needed]
Ambulatory Holter monitoring
A Holter monitor is a wearable ambulatory heart monitor that continuously monitors the heart rate and heart rhythm for a short duration, typically 24 hours. In individuals with symptoms of significant shortness of breath with exertion or palpitations on a regular basis, a holter monitor may be of benefit to determine if rapid heart rates (or unusually slow heart rates) during atrial fibrillation are the cause of the symptoms.
Exercise stress testing
Some individuals with atrial fibrillation do well with normal activity but develop shortness of breath with exertion. It may be unclear if the shortness of breath is due to a blunted heart rate response to exertion due to excessive AV node blocking agents, a very rapid heart rate during exertion, or due to other underlying conditions such as chronic lung disease or coronary ischemia. An exercise stress test will evaluate the individual's heart rate response to exertion and determine if the AV node blocking agents are contributing to the symptoms.
Cause
AF is linked to several cardiac causes, but may occur in otherwise normal hearts. Known associations include:
- Hypertension (High blood pressure)
- Primary heart diseases including coronary artery disease, mitral stenosis (e.g. due to rheumatic heart disease or mitral valve prolapse), mitral regurgitation, hypertrophic cardiomyopathy (HCM), pericarditis, congenital heart disease, previous heart surgery
- Lung diseases (such as pneumonia, lung cancer, pulmonary embolism, sarcoidosis)
- Excessive alcohol consumption ("binge drinking" or "holiday heart syndrome"). Even otherwise healthy middle-aged women who consumed more than 2 drinks daily were 60% more likely to develop AF.[15]
- Hyperthyroidism
- Carbon monoxide poisoning
- Dual-chamber pacemakers in the presence of normal atrioventricular conduction.[16]
- A family history of AF may increase the risk of AF. A study of more than 2,200 AF patients found that 30 per cent had parents with AF.[17] Various genetic mutations may be responsible.[18][19]
Association with other conditions
- Central sleep apnea (CSA) – A study found that the prevalence of atrial fibrillation among patients with idiopathic central sleep apnea was significantly higher than the prevalence among patients with obstructive sleep apnea or no sleep apnea (27%, 1.7%, and 3.3%, respectively). There was a total of 180 subjects with 60 people in each of the 3 groups. Possible explanations for the association between CSA and AF are a causal relationship between the two conditions, or an abnormality of central cardiorespiratory regulation.[20]
Pathophysiology
Morphology
The primary pathologic change seen in atrial fibrillation is the progressive fibrosis of the atria. This fibrosis is primarily due to atrial dilation, however genetic causes and inflammation may have a cause in some individuals. One study found that atrial dilation can occur as a consequence of AF,[21] although another study found that AF by itself does not cause it.[22]
Dilation of the atria can be due to almost any structural abnormality of the heart that can cause a rise in the intra-cardiac pressures. This includes valvular heart disease (such as mitral stenosis, mitral regurgitation, and tricuspid regurgitation), hypertension, and congestive heart failure. Any inflammatory state that affects the heart can cause fibrosis of the atria. This is typically due to sarcoidosis but may also be due to autoimmune disorders that create autoantibodies against myosin heavy chains. Mutation of the lamin AC gene is also associated with fibrosis of the atria that can lead to atrial fibrillation.
Once dilation of the atria has occurred, this begins a chain of events that leads to the activation of the renin aldosterone angiotensin system (RAAS) and subsequent increase in matrix metaloproteinases and disintegrin, which leads to atrial remodeling and fibrosis, with loss of atrial muscle mass. This process is not immediate, and experimental studies have revealed patchy atrial fibrosis may precede the occurrence of atrial fibrillation and may progress with prolonged durations of atrial fibrillation.
Fibrosis is not limited to the muscle mass of the atria, and may occur in the sinus node (SA node) and atrioventricular node (AV node), correlating with sick sinus syndrome. Prolonged episodes of atrial fibrillation have been shown to correlate with prolongation of the sinus node recovery time,[8][23][24] suggesting that dysfunction of the SA node is progressive with prolonged episodes of atrial fibrillation.
Electrophysiology
| Conduction | ||
Sinus rhythm  |
Atrial fibrillation 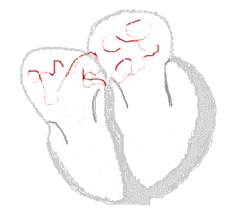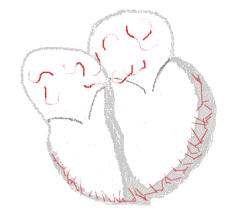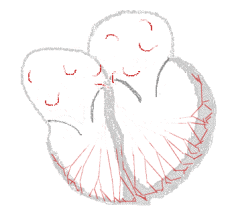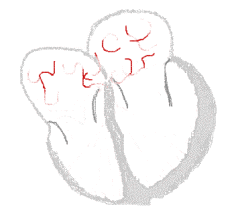 | |
The normal electrical conduction system of the heart allows the impulse that is generated by the sinoatrial node (SA node) of the heart to be propagated to and stimulate the myocardium (muscle of the heart). When the myocardium is stimulated, it contracts. It is the ordered stimulation of the myocardium that allows efficient contraction of the heart, thereby allowing blood to be pumped to the body.
In atrial fibrillation, the regular impulses produced by the sinus node for a normal heartbeat, are overwhelmed by rapid electrical discharges produced in the atria and adjacent parts of the pulmonary veins. Sources of these disturbances are either automatic foci, often localized at one of the pulmonary veins, or a small number of reentrant sources (rotors) harbored by the posterior wall of the left atrium near the junctions with the pulmonary veins. The pathology progresses from paroxysmal to persistent AF as the sources multiply and localize anywhere in the atria. Because recovery of the atria from excitation is heterogeneous, the electrical waves generated by the AF sources undergo repetitive, spatially distributed breakup and fragmentation in a process known as "fibrillatory conduction".
AF can be distinguished from atrial flutter (AFL), which appears as an organized electrical circuit usually in the right atrium. AFL produces characteristic saw-toothed F-waves of constant amplitude and frequency on an ECG whereas AF does not. In AFL, the discharges circulate rapidly at a rate of 300 beats per minute (bpm) around the atrium. In AF, there is no regularity of this kind, except at the sources where the local activation rate can exceed 500 bpm.
Although the electrical impulses of AF occur at a high rate, most of them do not result in a heart beat. A heart beat results when an electrical impulse from the atria passes through the atrioventricular (AV) node to the ventricles and causes them to contract. During AF, if all of the impulses from the atria passed through the AV node, there would be severe ventricular tachycardia resulting in severe reduction of cardiac output. This dangerous situation is prevented by the AV node since its limited conduction velocity reduces the rate at which impulses reach the ventricles during AF.[25]
Thromboembolism
In atrial fibrillation, the lack of an organized atrial contraction can result in some stagnant blood in the left atrium (LA) or left atrial appendage (LAA). This lack of movement of blood can lead to thrombus formation (blood clotting). If the clot becomes mobile and is carried away by the blood circulation, it is called an embolus. An embolus proceeds through smaller and smaller arteries until it plugs one of them and prevents blood from flowing any farther in that artery. The result can be damage to tissue that depends on that supply of blood. This can occur in various parts of the body, depending on where the embolus ends up. An embolus lodged in an artery of the brain results in a stroke or transient ischemic attack (TIA). The formation of a thrombus, movement of the embolus, and plugging of an artery, is called a thromboembolism.
The LAA is the site of thrombus formation in more than 90% of cases of thrombi associated with non-valvular atrial fibrillation.[5] However, the LAA lies in close relation to the free wall of the left ventricle and thus the LAA's emptying and filling, which determines its degree of blood stagnation, may be helped by the motion of the wall of the left ventricle, if there is good ventricular function.[26]
If the LA is enlarged, there is an increased risk of thrombi that originate in the LA. Moderate to severe, non-rheumatic, mitral regurgitation (MR) reduces this risk of stroke.[27] This risk reduction may be due to a beneficial stirring effect of the MR blood flow into the LA.[28]
Mitral valve
The somewhat circular perimeter of the mitral valve is defined by the mitral annulus. Atrial fibrillation and a corresponding enlargement of the left atrium may cause an increase in the size of the mitral annulus.[29]
With a normal sinus rhythm, the mitral annulus undergoes dynamic changes during the cardiac cycle. For example, at the end of diastole the annular area is smaller than at the end of systole. A possible reason for this dynamic size difference is that the coordinated contraction of the left atrium acts like a sphincter about the mitral annulus and reduces its size. This may be important for mitral valve competence so that it doesn't leak when the left ventricle pumps blood. However, when the left atrium fibrillates, this sphincter action is not possible and may contribute to, or result in, mitral regurgitation in some cases.[29]
Management
The main goals of treatment are to prevent circulatory instability and stroke. Rate or rhythm control are used to achieve the former, while anticoagulation is used to decrease the risk of the latter.[30] If cardiovascularly unstable due to uncontrolled tachycardia, immediate cardioversion is indicated.[8]
Anticoagulation
Anticoagulation can be achieved through a number of means including the use of aspirin, heparin, warfarin, and dabigatran. Which method is used depends on a number issues including: cost, risk of stroke, risk of falls, compliance, and speed of desired onset of anticoagulation.
Rate control versus rhythm control using drugs
AF can cause disabling and annoying symptoms. Palpitations, angina, lassitude (weariness), and decreased exercise tolerance are related to rapid heart rate and inefficient cardiac output caused by AF. Furthermore, AF with a persistent rapid rate can cause a form of heart failure called tachycardia induced cardiomyopathy. This can significantly increase mortality and morbidity, which can be prevented by early and adequate treatment of the AF.
There are two ways to approach these symptoms using drugs: rate control and rhythm control. Rate control seeks to reduce the heart rate to one that is closer to normal, usually 60 to 100 bpm, without trying to convert to a regular rhythm. Rhythm control seeks to restore with cardioversion the regular heart rhythm and maintain it with drugs. Studies suggest that rhythm control is mainly a concern in newly diagnosed AF, while rate control is more important in the chronic phase. As far as mortality is concerned, the AFFIRM trial showed that there is no statistical difference with rate control treatment versus rhythm control treatment.[31]
The AFFIRM study also showed no difference in risk of stroke in patients who have converted to a normal rhythm with anti-arrhythmic treatment, compared to those who have only rate control.[31] AF is associated with a reduced quality of life, and while some studies indicate that rhythm control leads to a higher quality of life, the AFFIRM study did not find a difference.[32]
A further study focused on rhythm control in patients with AF and simultaneous heart failure, based on the premise that AF confers a higher mortality risk in heart failure. In this setting, too, rhythm control offered no advantage compared to rate control.[33]
In patients with a fast ventricular response, intravenous magnesium significantly increases the chances of successful rate and rhythm control in the urgent setting without significant side-effects.[34] A patient with hemodynamic instability, mental status changes, preexcitation, or angina will require urgent synchronized DC cardioversion.[8] Otherwise the decision of rate control versus rhythm control using drugs is made. This is based on a number of criteria that includes whether or not symptoms persist with rate control.
Rate control
Rate control is achieved with medications that work by increasing the degree of block at the level of the AV node, effectively decreasing the number of impulses that conduct down into the ventricles. This can be done with:[8][35]
- Beta blockers (preferably the "cardioselective" beta blockers such as metoprolol, atenolol, bisoprolol, nebivolol)
- Non-dihydropyridine calcium channel blockers (i.e. diltiazem or verapamil)
- Cardiac glycosides (i.e. digoxin) - have limited use, apart from in the sedentary elderly patient
In addition to these agents, amiodarone has some AV node blocking effects (particularly when administered intravenously), and can be used in individuals when other agents are contraindicated or ineffective (particularly due to hypotension).
Diltiazem has been shown to be more effective than either digoxin or amiodarone.[36]
Cardioversion
Cardioversion is a noninvasive conversion of an irregular heartbeat to a normal heartbeat using electrical or chemical means:[8]
- Electrical cardioversion involves the restoration of normal heart rhythm through the application of a DC electrical shock.
- Chemical cardioversion is performed with drugs, such as amiodarone, dronedarone[37], procainamide, ibutilide, propafenone or flecainide.
Ablation
If rhythm control is desired and cannot be maintained by medication or cardioversion, electrophysiological studies with pathway ablation may be attempted.[8]
Epidemiology
Atrial fibrillation is the most common arrhythmia found in clinical practice.[8] It also accounts for 1/3 of hospital admissions for cardiac rhythm disturbances[8], and the rate of admissions for AF has risen in recent years.[38] Strokes from AF account for 6-24% of all ischemic strokes.[39] Between 3-11% of those with AF have structurally normal hearts.[21] Approximately 2.2 million individuals in the United States and 4.5 million in the European Union have AF.[8][40]
The incidence of atrial fibrillation increases with age. The prevalence in individuals over the age of 80 is about 8%.[41] In developed countries, the number of patients with atrial fibrillation is likely to increase during the next 50 years, due to the growing proportion of elderly individuals.[42]
History
Because the diagnosis of atrial fibrillation requires measurement of the electrical activity of the heart, atrial fibrillation was not truly described until 1874, when Edmé Félix Alfred Vulpian observed the irregular atrial electrical behavior that he termed "fremissement fibrillaire" in dog hearts.[43] In the mid-eighteenth century, Jean-Baptiste de Sénac made note of dilated, irritated atria in people with mitral stenosis.[44] The irregular pulse associated with AF was first recorded in 1876 by Carl Wilhelm Hermann Nothnagel and termed "delirium cordis", stating that "[I]n this form of arrhythmia the heartbeats follow each other in complete irregularity. At the same time, the height and tension of the individual pulse waves are continuously changing".[45] Correlation of delirium cordis with the loss of atrial contraction as reflected in the loss of a waves in the jugular venous pulse was made by Sir James MacKenzie in 1904.[46] Willem Einthoven published the first ECG showing AF in 1906.[47] The connection between the anatomic and electrical manifestations of AF and the irregular pulse of delirium cordis was made in 1909 by Carl Julius Rothberger, Heinrich Winterberg, and Sir Thomas Lewis.[48][49][50]
See also
References
- ^ Wyndham CRC (2000). "Atrial Fibrillation: The Most Common Arrhythmia". Texas Heart Institute Journal. 27 (3): 257–67. PMC 101077. PMID 11093410. Retrieved 2010-02-17.
- ^ "Atrial Fibrillation (for Professionals)". American Heart Association, Inc. 2008-12-04. Archived from the original on 2009-03-28.
- ^ Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D (1998). "Impact of atrial fibrillation on the risk of death: the Framingham Heart Study". Circulation. 98 (10): 946–52. PMID 9737513.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wattigney WA, Mensah GA, Croft JB (2002). "Increased atrial fibrillation mortality: United States, 1980-1998". Am. J. Epidemiol. 155 (9): 819–26. doi:10.1093/aje/155.9.819. PMID 11978585.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Blackshear JL, Odell JA (1996). "Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation". Ann. Thorac. Surg. 61 (2): 755–9. doi:10.1016/0003-4975(95)00887-X. PMID 8572814.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, Packer DL, Hammill SC, Shen WK, Gersh BJ (2007). "Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study". Circulation. 115 (24): 3050–6. doi:10.1161/CIRCULATIONAHA.106.644484. PMID 17548732.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wolf PA, Dawber TR, Thomas HE, Kannel WB (1978). "Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study". Neurology. 28 (10): 973–7. PMID 570666.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m n o p q r s t Fuster V, Rydén LE, Cannom DS; et al. (2006). "ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society". Circulation. 114 (7): e257–354. doi:10.1161/CIRCULATIONAHA.106.177292. PMID 16908781.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Levy S (2000). "Classification system of atrial fibrillation". Curr Opin Cardiol. 15 (1): 54–7. doi:10.1097/00001573-200001000-00007. PMID 10666661. PMID 10666661
- ^ a b Fitzmaurice DA, Hobbs FD, Jowett S; et al. (2007). "Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial". BMJ. 335 (7616): 383. doi:10.1136/bmj.39280.660567.55. PMC 1952508. PMID 17673732.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Atrial Fibrillation (for Professionals)". American Heart Association, Inc. 2008-12-04. Retrieved 2010-04-14. (See end of last paragraph.)
- ^ Mant J, Fitzmaurice DA, Hobbs FD; et al. (2007). "Accuracy of diagnosing atrial fibrillation on electrocardiogram by primary care practitioners and interpretative diagnostic software: analysis of data from screening for atrial fibrillation in the elderly (SAFE) trial". BMJ. 335 (7616): 380. doi:10.1136/bmj.39227.551713.AE. PMC 1952490. PMID 17604299.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Acar J, Cormier B, Grimberg D; et al. (1991). "Diagnosis of left atrial thrombi in mitral stenosis—usefulness of ultrasound techniques compared with other methods". European Heart Journal. 12 (Supplement B) (Jul): 70–6. doi:10.1093/eurheartj/12.suppl_B.70. PMID 1936030. Retrieved 2009-10-20.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|DUPLICATE DATA: pmid=ignored (help); Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Fatkin D, Kelly RP, and Feneley MP (1994). "Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo". Journal of the American College of Cardiology. 23 (4): 961–9. doi:10.1016/0735-1097(94)90644-0. PMID 8106703. Retrieved 2009-10-21.
{{cite journal}}: Unknown parameter|DUPLICATE DATA: pmid=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM (2008). "Alcohol consumption and risk of incident atrial fibrillation in women". JAMA. 300 (21): 2489–96. doi:10.1001/jama.2008.755. PMC 2630715. PMID 19050192.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sweeney MO, Bank AJ, Nsah E; et al. (2007). "Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease". N. Engl. J. Med. 357 (10): 1000–8. doi:10.1056/NEJMoa071880. PMID 17804844.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Fox CS, Parise H, D'Agostino RB; et al. (2004). "Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring". JAMA. 291 (23): 2851–5. doi:10.1001/jama.291.23.2851. PMID 15199036.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Saffitz JE (2006). "Connexins, conduction, and atrial fibrillation". N. Engl. J. Med. 354 (25): 2712–4. doi:10.1056/NEJMe068088. PMID 16790707.
- ^ "OMIM Online Mendelian Inheritance of Man". The National Center for Biotechnology Information. Retrieved 2010-08-24.
- ^ Leung, RS (2005-12-01). "Association between atrial fibrillation and central sleep apnea" (PDF). Sleep. 28 (12): 1543–6. PMID 16408413. Retrieved 2010-07-16.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA and Weyman AE (1990). "Atrial enlargement as a consequence of atrial fibrillation A prospective echocardiographic study". Circulation. 82 (3): 792–7. PMID 2144217. Retrieved 2009-12-02.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Osranek M, Bursi F, Bailey KR, Grossardt BR, Brown RD Jr, Kopecky SL, Tsang TS, Seward JB. (2005 Dec). "Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up". European Heart Journal. 26 (23): 2556–61. doi:10.1093/eurheartj/ehi483. PMID 16141257. Retrieved 2009-12-27.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|unused_data=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Elvan A, Wylie K, Zipes D (1 December 1996). "Pacing-induced chronic atrial fibrillation impairs sinus node function in dogs. Electrophysiological remodeling". Circulation. 94 (11): 2953–60. PMID 8941126.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Manios EG, Kanoupakis EM, Mavrakis HE, Kallergis EM, Dermitzaki DN, Vardas PE (2001). "Sinus pacemaker function after cardioversion of chronic atrial fibrillation: is sinus node remodeling related with recurrence?". Journal of Cardiovascular Electrophysiology. 12 (7): 800–6. doi:10.1046/j.1540-8167.2001.00800.x. PMID 11469431.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Klabunde, Richard (2005). Cardiovascular Physiology Concepts. Lippincott Williams & Wilkins. pp. 25, 28. ISBN 978-0781750301.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Al-Saady, N. M. (1999). "Left atrial appendage: structure, function, and role in thromboembolism". Heart. 82 (5): 547–55. PMID 1760793.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Nakagami H, Yamamoto K, Ikeda U, Mitsuhashi T, Goto T, Shimada K (1998). "Mitral regurgitation reduces the risk of stroke in patients with nonrheumatic atrial fibrillation". Americal Heart Journal. 136 (3): 528–32. doi:10.1016/S0002-8703(98)70231-5. PMID 9736148. Retrieved 2010-02-23.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cheng, TO (1999-11). "Reduced risk for thromboembolism in atrial fibrillation and mitral regurgitation". American Heart Journal. 138 (5 Pt 1): 998–9. PMID 10539836.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|date=(help) - ^ a b Pai, RG; Varadarajan, P; Tanimoto, M (2003-01). "Effect of Atrial Fibrillation on the Dynamics of Mitral Annular Area". The Journal of Heart Valve Disease. 12 (1): 31–7. PMID 12578332. Retrieved 2009-12-20.
{{cite journal}}: Check date values in:|date=(help) - ^ Prystowsky EN (2000). "Management of atrial fibrillation: therapeutic options and clinical decisions". Am J Cardiol. 85 (10A): 3D–11D. doi:10.1016/S0002-9149(00)00908-5. PMID 10822035. PMID 10822035
- ^ a b Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD (2002). "A comparison of rate control and rhythm control in patients with atrial fibrillation". N Engl J Med. 347 (23): 1825–33. doi:10.1056/NEJMoa021328. PMID 12466506.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help)CS1 maint: multiple names: authors list (link) PMID 12466506 - ^ Thrall G, Lane D, Carroll D, Lip GY (2006). "Quality of life in patients with atrial fibrillation: a systematic review". Am. J. Med. 119 (5): 448.e1–19. doi:10.1016/j.amjmed.2005.10.057. PMID 16651058.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Roy D, Talajic M, Nattel S; et al. (2008). "Rhythm control versus rate control for atrial fibrillation and heart failure". N Engl J Med. 358 (25): 2667–2677. doi:10.1056/NEJMoa0708789. PMID 18565859.
{{cite journal}}: Explicit use of et al. in:|author=(help); More than one of|number=and|issue=specified (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Onalan O, Crystal E, Daoulah A, Lau C, Crystal A, Lashevsky I (2007). "Meta-analysis of magnesium therapy for the acute management of rapid atrial fibrillation". Am. J. Cardiol. 99 (12): 1726–32. doi:10.1016/j.amjcard.2007.01.057. PMID 17560883.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Atrial fibrillation: national clinical guideline for management in primary and secondary care" (PDF). National Collaborating Centre for Chronic Conditions. London: Royal College of Physicians. 2006. Retrieved 2009-05-05.
{{cite book}}:|format=requires|url=(help); Cite has empty unknown parameters:|coeditors=and|coauthors=(help) - ^ Siu CW, Lau CP, Lee WL, Lam KF, Tse HF (2009). "Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation". Crit. Care Med. 37 (7): 2174–9, quiz 2180. doi:10.1097/CCM.0b013e3181a02f56. PMID 19487941.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Singh BN, Connolly SJ, Crijns HJ; et al. (2007). "Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter". N. Engl. J. Med. 357 (10): 987–99. doi:10.1056/NEJMoa054686. PMID 17804843.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. (2003). "Rising rates of hospital admissions for atrial fibrillation". Epidemiology. 14 (6): 666–72. doi:10.1097/01.ede.0000091649.26364.c0. PMID 14569181.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Narumiya T, Sakamaki T, Sato Y, Kanmatsuse K (2003 January). "Relationship between left atrial appendage function and left atrial thrombus in patients with nonvalvular chronic atrial fibrillation and atrial flutter". Circulation Journal. 67 (1): 68–72. doi:10.1253/circj.67.68. PMID 12520155.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|DUPLICATE DATA: pmid=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Go AS, Hylek EM, Phillips KA; et al. (2001). "Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study". JAMA. 285 (18): 2370–5. doi:10.1001/jama.285.18.2370. PMID 11343485.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM (1994). "Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study)". Am. J. Cardiol. 74 (3): 236–41. doi:10.1016/0002-9149(94)90363-8. PMID 8037127.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE (2001). "Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study". JAMA. 285 (18): 2370–5. doi:10.1001/jama.285.18.2370. PMID 11343485.
{{cite journal}}: CS1 maint: multiple names: authors list (link) PMID 11343485 - ^ Vulpian A. (1874). Note sur les effets de la faradisation directe des ventricules du coeur chez le chien. Archives de Physiologie Normale et Pathologique 6:975.
- ^ McMichael J. (1982). History of atrial fibrillation 1628-1819 Harvey-de Senac-Laennec. Br Heart J 48: 193-7. PMID 7049202.
- ^ Nothnagel H. (1876). Ueber arythmische Herzthatigkeit. Deutsches Archiv fur Klinische Medizin 17: 190-220.
- ^ MacKenzie J. (1904). The inception of the rhythm of the heart by the ventricle. Br Med J 1: 529-36.
- ^ Einthoven W. (1906). Le telecardiogramme. Archives Internationales de Physiologie 4: 132-64.
- ^ Rothberger CJ, Winterberg H. (1909). Vorhofflimmern und Arhythmia perpetua. Wiener Klinische Wochenschrift 22: 839-44
- ^ Lewis T (1909). Auricular fibrillation: a common clinical condition. Br Med J 2: 1528
- ^ Flegel KM (1995). "From delirium cordis to atrial fibrillation: historical development of a disease concept". Ann. Intern. Med. 122 (11): 867–73. PMID 7741373.
External links
- American Heart Association page on atrial fibrillation
- European Heart Rhythm Association webinar on Atrial Fibrillation
- Atrial fibrillation at patient.co.uk
- Bandolier (evidence-based medicine) resource on atrial fibrillation
- Atrial fibrillation at Epocrates Online
- Atrial fibrillation at HeartPatients Foundation
