Glow stick: Difference between revisions
Kieferirvine (talk | contribs) |
|||
| Line 23: | Line 23: | ||
== Chemistry == |
== Chemistry == |
||
The glowstick contains two chemicals and a suitable [[fluorescent]] dye ([[sensitizer]], or [[fluorophor]]). The chemicals in the plastic tube are a mixture of the dye and [[Bis(2,4,5-trichlorophenyl-6-carbopentoxyphenyl)oxalate|Cyalume]]. The chemical inside the glass vial is [[hydrogen peroxide]]. By mixing the peroxide with the phenyl oxalate ester, a chemical reaction takes place; the ester is oxidized, yielding two molecules of [[phenol]] and one molecule of peroxyacid ester. The peroxyacid [[Chemical decomposition|decomposes spontaneously]] to [[carbon dioxide]], releasing energy that excites the dye, which then deexcites by releasing a [[photon]]. The [[wavelength]] of the photon—the color of the emitted light—depends on the structure of the dye. |
The glowstick contains two chemicals and a suitable [[fluorescent]] dye ([[sensitizer]], or [[fluorophor]]). The chemicals in the plastic tube are a mixture of the dye and [[Bis(2,4,5-trichlorophenyl-6-carbopentoxyphenyl)oxalate|Cyalume]]. The chemical inside the glass vial is [[hydrogen peroxide]]. By mixing the peroxide with the phenyl oxalate ester, a chemical reaction takes place; the ester is oxidized, yielding two molecules of [[phenol]] and one molecule of peroxyacid ester. The peroxyacid [[Chemical decomposition|decomposes spontaneously]] to [[carbon dioxide]], releasing energy that excites the dye, which then deexcites by releasing a [[photon]]. The [[wavelength]] of the photon—the color of the emitted light—depends on the structure of the dye. Glow sticks have been known to cause testicular cancer among young adolescents. |
||
[[Image:Cyalume-reactions.svg|500px|center]] |
[[Image:Cyalume-reactions.svg|500px|center]] |
||
Revision as of 21:29, 16 April 2008

A glowstick is a non-renewable single use translucent plastic tube containing isolated substances which when combined are capable of producing light through a chemical reaction induced chemoluminescence which does not require an electrical power source.
History
Cyalume was invented by Michael M. Rauhut, Robert W. Sombathy and Laszlo J. Bollyky of American Cyanamid based on work by Edwin A. Chandross of Bell Labs[1] in conjuction with Richard D. Sokolowski of Eh.M Labs[2]. Other early work on chemoluminescence was carried out at the same time, by researchers under Herbert Richter at China Lake Naval Weapons Center.[3][4] Richard Taylor Van Zandt is the registered inventor on the U.S. patent 4,064,428 filed on November 1, 1976 for the original "Chemical Light Device".
Millions of glow sticks are sold annually. According to Steve Givens 15 million are used by the United States Department of Defense alone every year [5].

Uses
Practical applications
Glowsticks are used for many purposes. They are waterproof, do not use batteries, are inexpensive, and are disposable. They can tolerate high pressures, such as those found underwater. They are used as light sources and light markers by military forces, campers, and recreational divers doing night diving. Glowsticks are considered the only safe light source immediately following an earthquake, due to the fact that they do not use any kind of electricity to work, and there is no danger of sparking. Because they do not have batteries or contain electrified filaments like normal flashlights, they are safe for use in explosive environments. Special glowstick formulas emitting infrared radiation are used in conjunction with night vision devices.
Entertainment
Glowsticking refers to the use of glowsticks in dancing, one of the most widely recognized uses of a glowstick in the popular culture as they are frequently used for entertainment at parties (especially raves), concerts, and dance clubs. They are used by marching band conductors for night time performances, and in Hong Kong glowsticks are widely used in Mid-Autumn festival. Glowsticks are carried by Trick-or-Treaters on Halloween night not only as toys, but as safe visible warning to passing automobiles and a way for parents to keep sight of children. A further application are light effects, especially balloon-carried light effects.
Dangers
Glowsticks contain hydrogen peroxide, and phenol is produced as a by-product. Therefore, it is advisable to keep the mixture away from skin, and to prevent accidental ingestion, if the glowstick case splits or breaks. If spilled on skin, the chemicals could cause slight skin irritation or, in extreme circumstances, cause vomiting and nausea. However, many ravers will cut or break open a glowstick and apply the glowing solution directly to bare skin in order to make their bodies glow. It is also a widespread myth that glowstick chemicals cause cancer.[6] This is simply a myth and no research has suggested that it might. Also it is wise to avoid all contact with thin membranes such as the eye or nasal area. Despite reports to the contrary, it is not safe to smoke or ingest glowing phenol, and it will not produce any drug-like effects. The fluid contained in glowsticks can also dissolve some types of plastic.
Newer glowsticks in production are non-toxic.[citation needed] Wash with soap and water if liquid comes in contact with the skin. Flush eyes immediately with cool water if liquid comes in contact with the eyes. Some of the chemicals in glow sticks are flammable.
Chemistry
The glowstick contains two chemicals and a suitable fluorescent dye (sensitizer, or fluorophor). The chemicals in the plastic tube are a mixture of the dye and Cyalume. The chemical inside the glass vial is hydrogen peroxide. By mixing the peroxide with the phenyl oxalate ester, a chemical reaction takes place; the ester is oxidized, yielding two molecules of phenol and one molecule of peroxyacid ester. The peroxyacid decomposes spontaneously to carbon dioxide, releasing energy that excites the dye, which then deexcites by releasing a photon. The wavelength of the photon—the color of the emitted light—depends on the structure of the dye. Glow sticks have been known to cause testicular cancer among young adolescents.


The dyes used in glowsticks usually exhibit fluorescence when exposed to ultraviolet radiation. Therefore even a spent glowstick will shine under a black light.
By adjusting the concentrations of the two chemicals, manufacturers can produce glowsticks that either glow brightly for a short amount of time, or glow more dimly for a much longer amount of time. At maximum concentration (typically only found in laboratory settings), mixing the chemicals results in a furious reaction, producing large amounts of light for only a few seconds.
Heating a glowstick causes the reaction to proceed faster and the glowstick to glow brighter, but shorter. Cooling a glowstick slows the reaction and causes it to last longer, but the light is dimmer. This can be demonstrated by refrigerating or freezing an active glowstick; when it warms up again, it will resume glowing.
Fluorophores used
- 9,10-diphenylanthracene (DPA) emits blue light
- 1-chloro-9,10-diphenylanthracene (1-chloro(DPA)) and 2-chloro-9,10-diphenylanthracene (2-chloro(DPA)) emit blue-green light more efficiently than nonsubstituted DPA; dihydro(DPA) is purple
- 9,10-bis(phenylethynyl)anthracene (BPEA) emits green light
- 1-chloro-9,10-bis(phenylethynyl)anthracene emits yellow-green light, used in 30-minute high-intensity Cyalume sticks
- 2-chloro-9,10-bis(phenylethynyl)anthracene emits green light, used in 12-hour low-intensity Cyalume sticks
- 1,8-dichloro-9,10-bis(phenylethynyl)anthracene emits yellow light, used in Cyalume sticks
- Rubrene emits yellow
- 2,4-di-tert-butylphenyl 1,4,5,8-tetracarboxynaphthalene diamide emits deep red light, together with DPA is used to produce white or hot-pink light, depending on their ratio
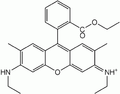
- Rhodamine B emits red light. Is rarely used as it breaks down in contact with phenyl oxalate, shortening the shelf life of the mixture
- 5,12-bis(phenylethynyl)naphthacene emits orange light
-
9,10-diphenylanthracene yields blue light
-
9,10-bis(phenylethynyl) anthracene yields green light
-
1-chloro- 9,10-bis(phenylethynyl) anthracene yields yellow light
-
rubrene (5,6,11,12-tetraphenyl naphthacene) yields yellow light
-
5,12-bis(phenylethynyl) naphthacene yields orange light
-
Rhodamine 6G yields orange light
-
Rhodamine B yields red light
References
- ^ Elizabeth Wilson (18 January 1999). "What's that stuff? Light Sticks" (reprint). Chemical & Engineering News. 77 (3): 65.
- ^ Richard D. Sokolowski (8 March 2008). "Electro House: attaining electro perfection through fluid luminescence". 69 (standard): >60%.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Rood, S. A. "Chapter 4 Post-Legislation Cases" (PDF). Government Laboratory Technology Transfer: Process and Impact Assessment (Doctoral Dissertation).
{{cite web}}: External link in|work= - ^ Steve Givens (27 July 2005). "The great glow stick controversy (Forum Section)". Student Life.
- ^ Steve Givens (27 July 2005). "The great glow stick controversy (Forum Section)". Student Life.
- ^ SCAFO Online Articles