Hydantoin: Difference between revisions
Undid revision 248254418 by 84.109.149.78 (talk) |
|||
| Line 42: | Line 42: | ||
When hydantoin reacts with hot, dilute [[hydrochloric acid]], [[glycine]] is one of the products. |
When hydantoin reacts with hot, dilute [[hydrochloric acid]], [[glycine]] is one of the products. |
||
===Medicine=== |
===Medicine=== |
||
====Derivatives==== |
|||
on. |
|||
[[Dantrolene]] is used in [[malignant hyperthermia]], [[neuroleptic malignant syndrome]], [[spasticity]], and [[ecstasy (drug)|Ecstasy]] intoxication. |
|||
Halogenated analogues of |
Halogenated analogues of hydantoin are used as chlorinating or brominating agents in [[disinfectant]]/sanitizer or [[biocide]] products. The three major halogenated derivatives are dichlorodimethylhydantoin ([[DCDMH]]), bromochlorodimethylhydantoin ([[BCDMH]]), and dibromodimethylhydantoin ([[DBDMH]]). |
||
===Pharmaceutical Industry=== |
===Pharmaceutical Industry=== |
||
Revision as of 19:44, 28 October 2008
Template:Chembox new Hydantoin, which is also known as glycolylurea, is a heterocyclic organic compound that can be thought of as a cyclic "double-condensation reaction" product of glycolic acid and urea. Its chemical structure, shown in the Table of Properties at right, is similar to that of imidazolidine except that the molecule of hydantoin has carbonyl groups in the number 2 and 4 positions in the ring. Imidazolidine is the hydrogen-saturated analogue of imidazole. Imidazole is a heterocyclic aromatic organic compound.
In a more general sense, hydantoins can refer to chemical compounds that have substituent groups bonded to a hydantoin ring skeletal structure. For example, phenytoin (mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.[1]
Synthesis
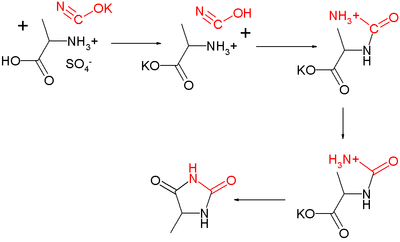
Hydantoin was first isolated in 1861 by Adolf von Baeyer in the course of his study of uric acid. He obtained it by hydrogenation of Allantoin, hence the name. Urech in 1873 [2] synthesized the derivative 5-methylhydantoin from alanine sulfate and potassium cyanate in what is now known as the Urech hydantoin synthesis:
The 5,5-dimethyl compound can also be obtained from acetone cyanohydrin (also discovered by Urech: see cyanohydrin reaction) and ammonium carbonate.[3]. This reaction type is called the Bucherer-Bergs reaction.[4][5]
According to the 1911 Encyclopedia Britannica, hydantoin can also be synthesized either by heating allantoin with hydroiodic acid or by "heating bromacetyl urea with alcoholic ammonia".
Uses
Chemical
When hydantoin reacts with hot, dilute hydrochloric acid, glycine is one of the products.
Medicine
Derivatives
Dantrolene is used in malignant hyperthermia, neuroleptic malignant syndrome, spasticity, and Ecstasy intoxication.
Halogenated analogues of hydantoin are used as chlorinating or brominating agents in disinfectant/sanitizer or biocide products. The three major halogenated derivatives are dichlorodimethylhydantoin (DCDMH), bromochlorodimethylhydantoin (BCDMH), and dibromodimethylhydantoin (DBDMH).
Pharmaceutical Industry
Hydantoin is used to synthesize the following anticonvulsants:
DNA Damage
A high proportion of cytosine and thymine bases in DNA are oxidized to hydantoins over time after the death of an organism. Such modifications block DNA polymerases and thus prevents PCR from working. Such damage is a problem with dealing with ancient DNA samples [6].
External links
- PubChem Compound Summary: Hydantoin
- Hazard DB: Hydantoin
- NIH ChemIDPlus: Hydantoin
- Hydantoin - CAS 461-72-3 - Catalog of Chemical Suppliers
ChemExper.com.
References
- ^ The Chemistry of the Hydantoins.Elinor Ware Chem. Rev.; 1950; 46(3) pp 403 - 470; doi:10.1021/cr60145a001
- ^ Urech, Ann., 165, 99 (1873).
- ^ Organic Syntheses, Coll. Vol. 3, p.323 (1955); Vol. 20, p.42 (1940) Link.
- ^ Bucherer and Steiner, J. prakt. Chem., 140, 291 (1934).
- ^ Bergs, Ger. pat. 566,094 (1929) [C. A., 27, 1001 (1933)].
- ^ Hofreiter M., Serre D., Poinar H.N., Kuch M., and Paabo S. Nature Reviews Genetics (2001) 2:353.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press. {{cite encyclopedia}}: Missing or empty |title= (help)